In situ synthesis of copper nanowire array material and its preparation method and application
A technology of copper nanowires and in-situ synthesis, which is applied in nanotechnology, nanotechnology, chemical instruments and methods, etc., can solve the problem of unstable catalysts, suitable deuterium donors, and the inability to accurately control the deuterium position and number, and deuterium rate No high problems, achieve the effect of maintaining shape and performance, low cost, and high deuterium substitution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Step 1: The copper sheet (3cm*1cm*0.1mm) was ultrasonicated in acetone and 3.0M HCl solution for 15min respectively, then rinsed with distilled water and ethanol respectively, and dried with cold air in the air for subsequent use;
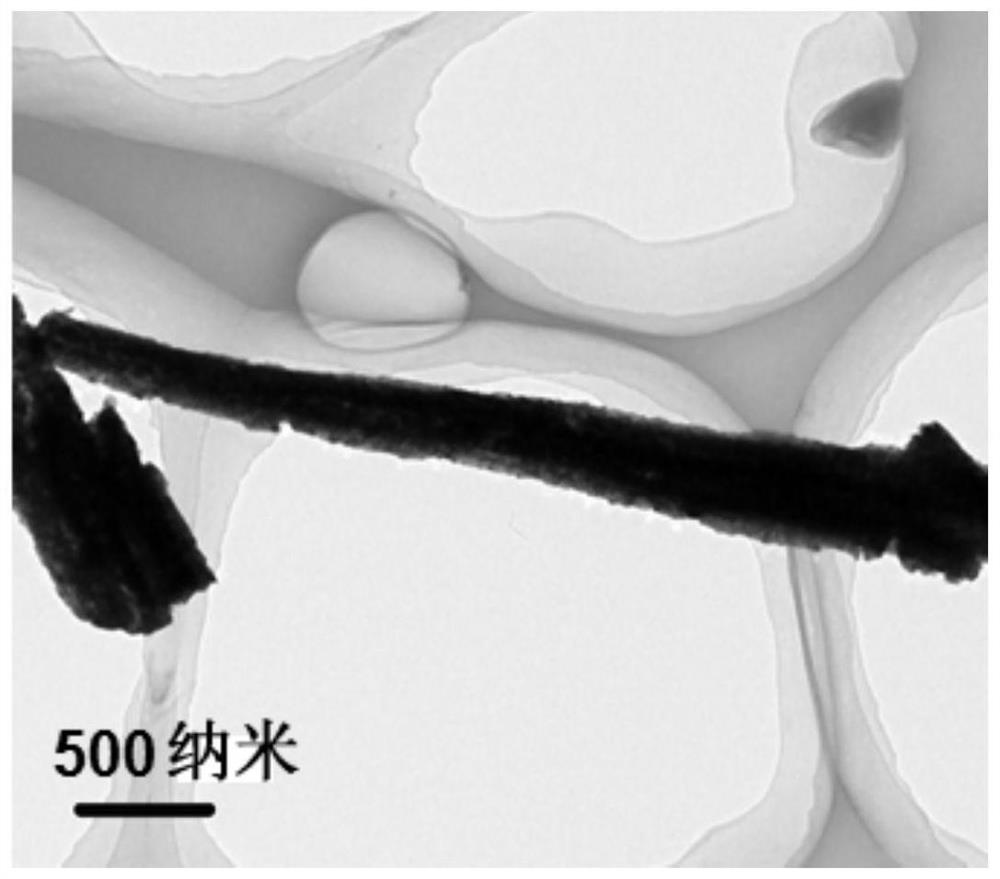
[0053] In step 2, two copper sheets obtained in step 1 were taken out and placed in a single-chamber reaction cell, respectively the anode and the cathode, and the electrodeposition reaction was carried out with 3M NaOH solution as the electrolyte. 2 The reaction was carried out for 6 min under the current density of 100 Å to form a dark blue copper hydroxide nanowire array precursor;
[0054] Step 3, placing the dark blue copper hydroxide precursor prepared in step 2 in a quartz tube with a diameter of 6 mm, calcining at 150° C. for 3 hours, and heating at a rate of 2° C. / min to obtain a copper oxide nanowire array;
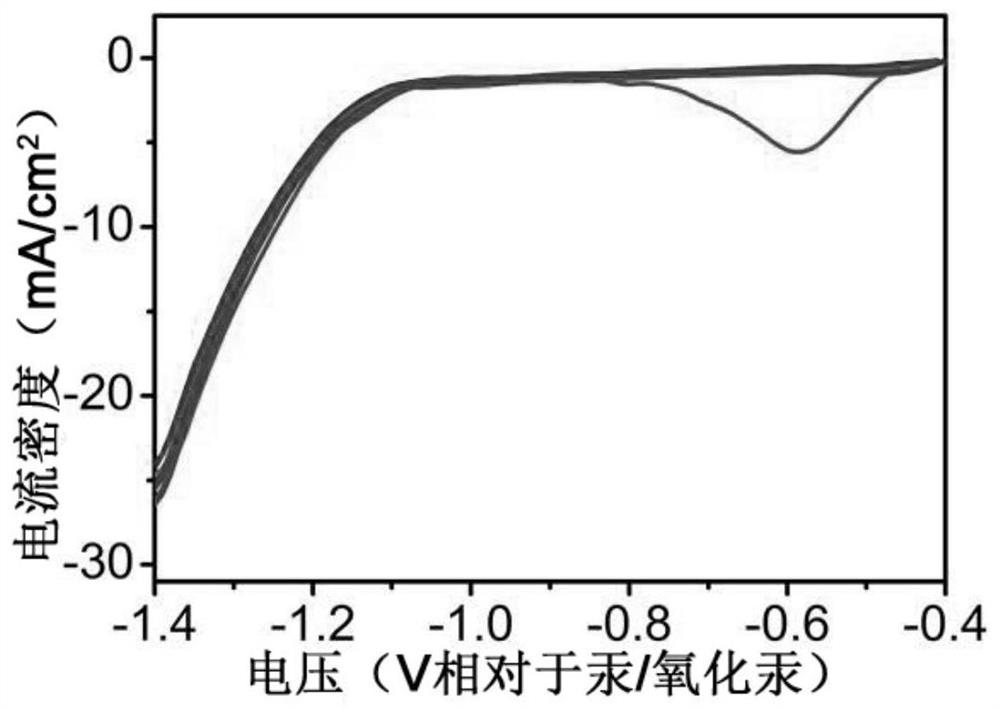
[0055] Step 4, a two-chamber separated electrolytic cell was used, using the copper nanowire array as the working electrode,...
Embodiment 2
[0057] Step 1: The copper sheet (3cm*1cm*0.1mm) was ultrasonicated in acetone and 3.0M HCl solution for 15min respectively, then rinsed with distilled water and ethanol respectively, and dried with cold air in the air for subsequent use;
[0058] In step 2, two copper sheets obtained in step 1 were taken out and placed in a single-chamber reaction cell, respectively the anode and the cathode, and the electrodeposition reaction was carried out with 3M NaOH solution as the electrolyte. 2 The reaction was carried out for 6 min under the current density of 100 Å to form a dark blue copper hydroxide nanowire array precursor;
[0059] Step 3, placing the dark blue copper hydroxide precursor prepared in step 2 in a quartz tube with a diameter of 6 mm, calcining at 100° C. for 4 hours, and heating at a rate of 2° C. / min to obtain a copper oxide nanowire array;
[0060] Step 4, a two-chamber separated electrolytic cell was used, using the copper nanowire array as the working electrode,...
Embodiment 3
[0062] Step 1: The copper sheet (3cm*1cm*0.1mm) was ultrasonicated in acetone and 3.0M HCl solution for 15min respectively, then rinsed with distilled water and ethanol respectively, and dried with cold air in the air for subsequent use;
[0063] In step 2, two copper sheets obtained in step 1 were taken out and placed in a single-chamber reaction cell, respectively the anode and the cathode, and the electrodeposition reaction was carried out with 3M NaOH solution as the electrolyte. 2 The reaction was carried out for 6 min under the current density of 100 Å to form a dark blue copper hydroxide nanowire array precursor;
[0064] Step 3, placing the dark blue copper hydroxide precursor prepared in step 2 in a quartz tube with a diameter of 6 mm, calcining at 250° C. for 2 hours, and heating at a rate of 2° C. / min to obtain a copper oxide nanowire array;
[0065] Step 4, a two-chamber separated electrolytic cell was used, using the copper nanowire array as the working electrode,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com