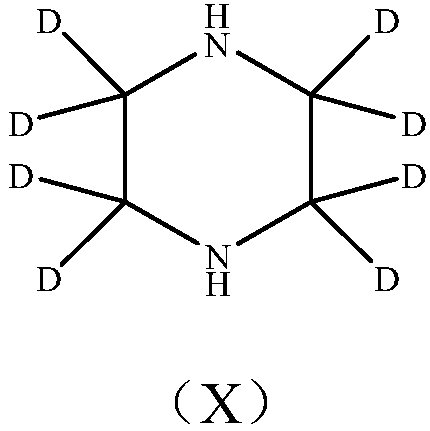
Deuterated piperazine and preparation method thereof
A technology of deuterated piperazine and piperazine, applied in the field of isotope chemicals and preparation, can solve problems such as undiscovered preparation method of deuterated piperazine, and achieve the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The preparation method of deuterated piperazine, comprises the steps,
[0033] (1) Put piperazine in the liner of polytetrafluoroethylene reactor, then add deuterated methanol CD 3 OD;
[0034] (2) Put the above-mentioned liner into the supporting stainless steel kettle body, and tighten the lid of the kettle with bolts;
[0035] (3) Continuous heating at high temperature, the heating temperature is controlled at 100°C, and the heating time is controlled at 96h;
[0036] (4) After the heating is completed, the polytetrafluoroethylene reactor is naturally cooled to room temperature, then the reaction system in the liner is transferred to a single-necked flask, and concentrated under reduced pressure until there is no solvent to obtain deuterated piperazine.
[0037] The deuterated piperazine prepared according to the technical solution of the present embodiment adopts gas chromatography to measure the deuterated piperazine purity to be 91.76%.
Embodiment 2
[0039] The preparation method of deuterated piperazine, comprises the steps,
[0040] (1) Put piperazine in the liner of polytetrafluoroethylene reactor, then add deuterated methanol CD 3 OD;
[0041] (2) Put the above-mentioned liner into the supporting stainless steel kettle body, and tighten the lid of the kettle with bolts;
[0042] (3) Continuous heating at high temperature, the heating temperature is controlled at 150°C, and the heating time is controlled at 72h;
[0043] (4) After the heating is completed, the polytetrafluoroethylene reactor is naturally cooled to room temperature, then the reaction system in the liner is transferred to a single-necked flask, and concentrated under reduced pressure until there is no solvent to obtain deuterated piperazine.
[0044] The deuterated piperazine prepared according to the technical solution of the present embodiment adopts gas chromatography to determine that the deuterated piperazine purity is 92.44%.
Embodiment 3
[0046] The preparation method of deuterated piperazine, comprises the steps,
[0047] (1) Put piperazine in the liner of polytetrafluoroethylene reactor, then add deuterated methanol CD 3 OD;
[0048] (2) Put the above-mentioned liner into the supporting stainless steel kettle body, and tighten the lid of the kettle with bolts;
[0049] (3) Continuous heating at high temperature, the heating temperature is controlled at 200°C, and the heating time is controlled at 48h;
[0050] (4) After the heating is completed, the polytetrafluoroethylene reactor is naturally cooled to room temperature, then the reaction system in the liner is transferred to a single-necked flask, and concentrated under reduced pressure until there is no solvent to obtain deuterated piperazine.
[0051] The purity of the deuterated piperazine obtained according to the technical solution of the present embodiment is 92.46% by gas chromatography.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com