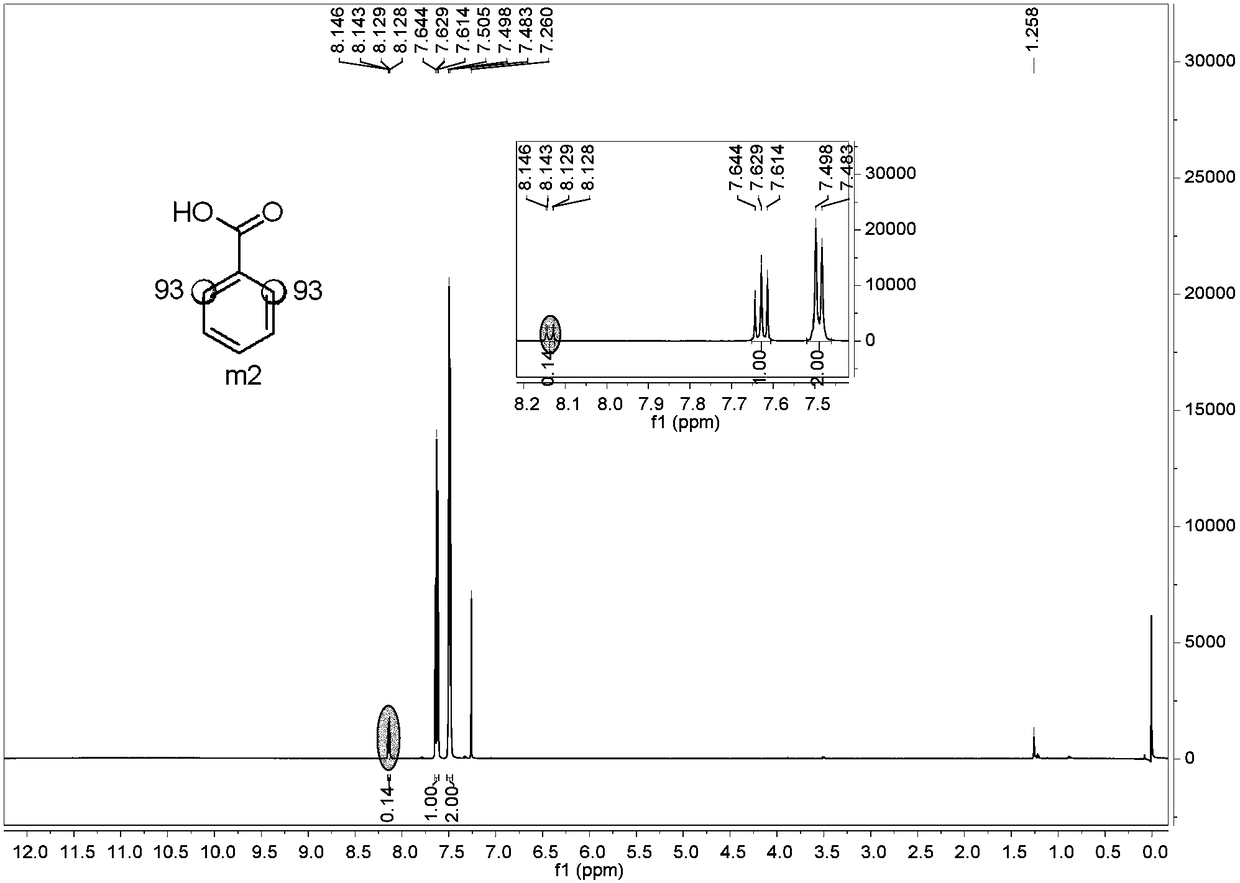
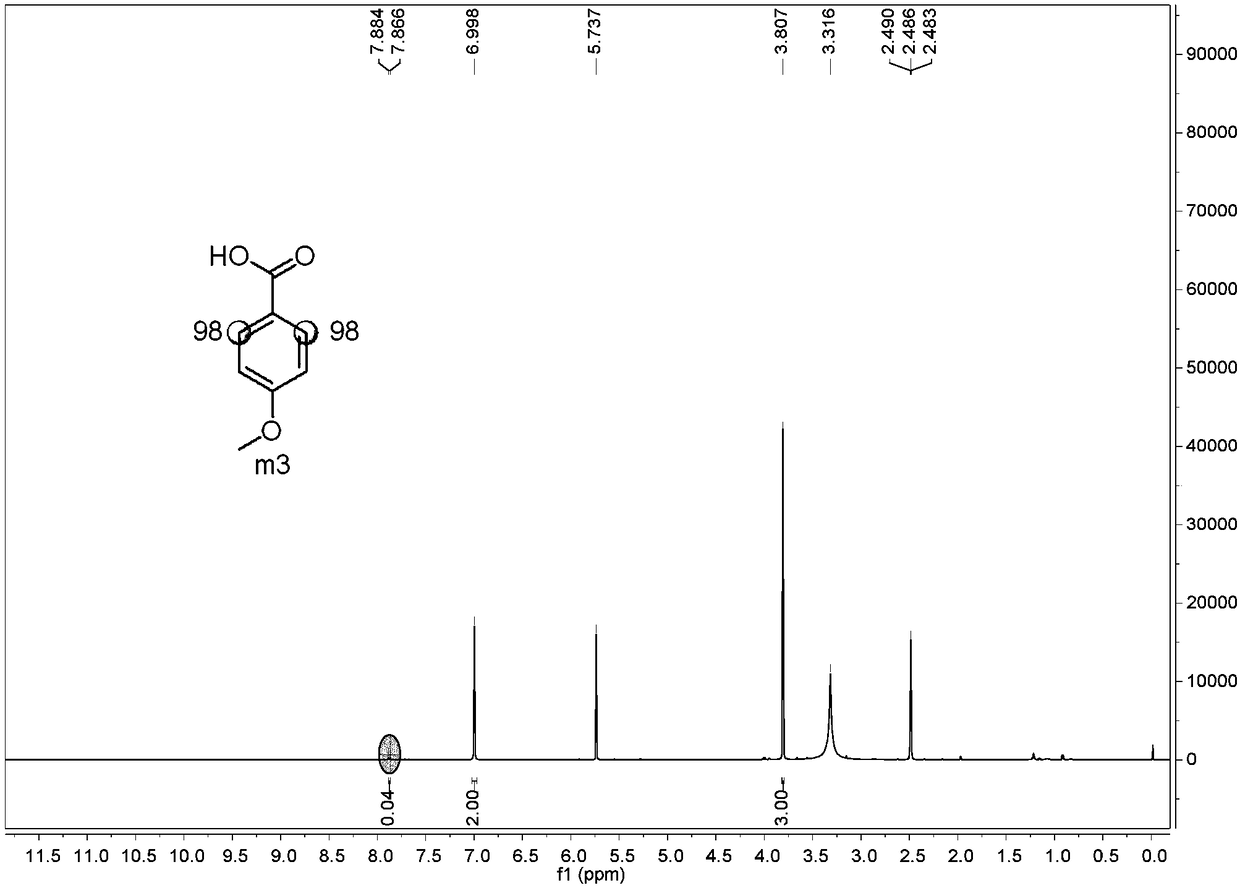
Preparation method of o-position deuterated benzoic acid compound
A technology of deuterated benzoic acid and compounds, which is applied in the field of synthesis of deuterated compounds, can solve the problems of low deuterated rate of reaction, difficult production and supply, low deuterated rate of ortho-deuterated o-toluic acid, etc. Achieve the effect of good economy, wide application and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
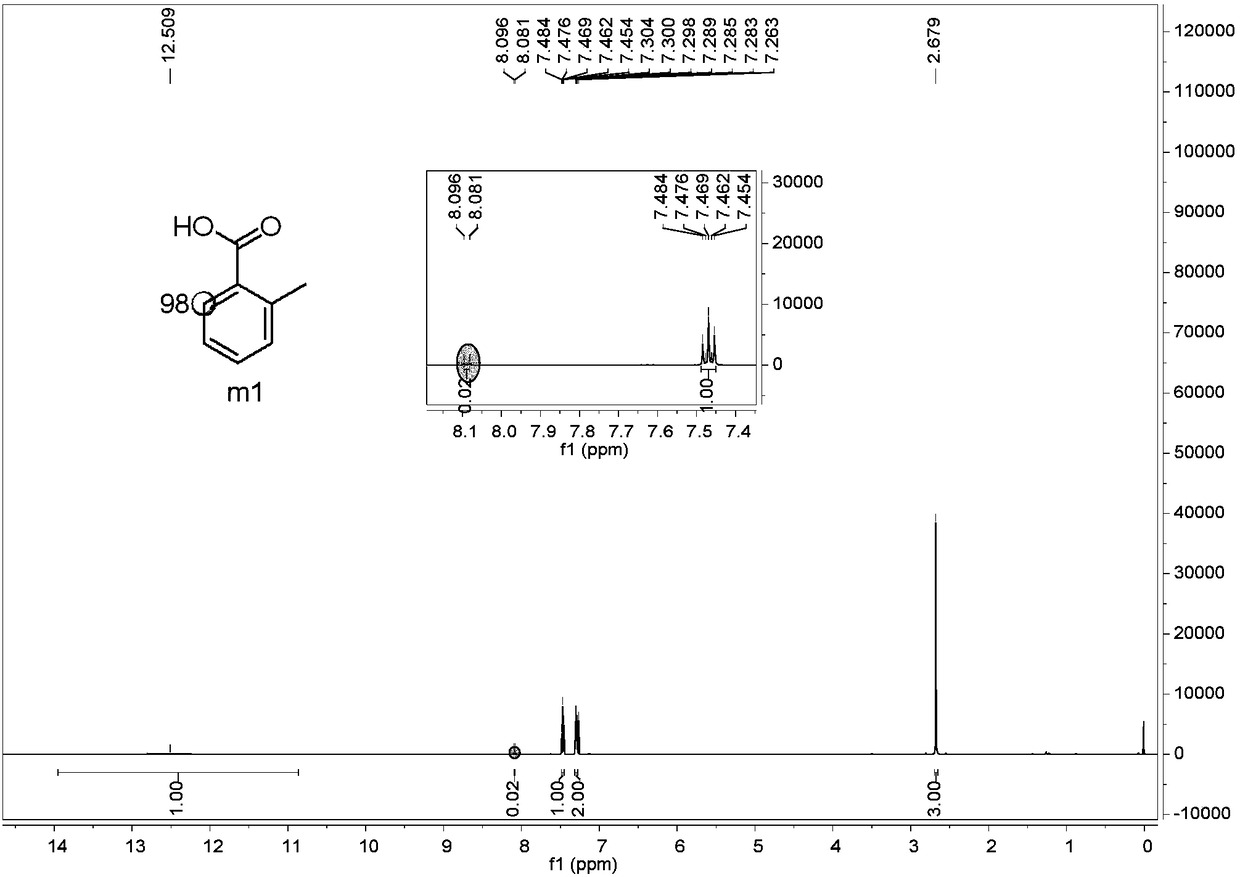
[0057] The preparation method of embodiment 1-1,6-deuterium-2-methylbenzoic acid (m1), carries out the following steps successively:
[0058] 1) Weigh 0.1312g (0.5mmol, 1.0eq.) of 8-aminoquinoline o-toluic acid amide, 0.0224g (0.1mmol, 0.2eq.) of palladium acetate and 2mL of heavy water in a sealed tube. After sealing Reaction in an oil bath at 140°C for 48h.
[0059] 2), after the reaction solution obtained in step 1) was cooled to room temperature, 15mL of water was added, and ethyl acetate (3×20mL) was added for extraction, the organic phases were combined, washed with saturated brine (30mL), and then washed with anhydrous sodium sulfate 4g was dried, concentrated under reduced pressure by a rotary evaporator, the concentration temperature was 30-40°C, the vacuum degree was not lower than -0.07MPa, and concentrated until there was no obvious droplet dripping to obtain a concentrate;
[0060] 3), the concentrate obtained in step 2) is separated and purified by silica gel ch...
Embodiment 1-2
[0069] Example 1-2, changing the 140°C locking reaction to 120°C locking reaction, the rest is the same as Example 1-1, and the deuteration rate is 81%.
[0070] Example 1-3, changing the 140°C locking reaction to 160°C locking reaction, the rest is the same as Example 1-1, and the deuteration rate is 96%.
[0071] Example 1-4, changing the 140°C locking reaction to a reflux device reflux reaction, the rest is the same as Example 1-1, and the deuteration rate is 96%.
Embodiment 1-5
[0072] Example 1-5, change 0.2eq. palladium acetate to 0.1eq., the rest are the same as Example 1-1, the deuteration rate is 91%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com