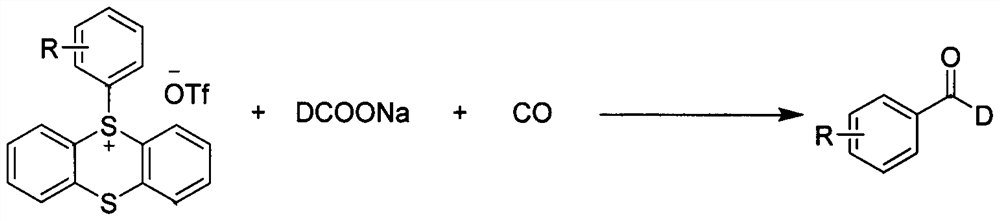
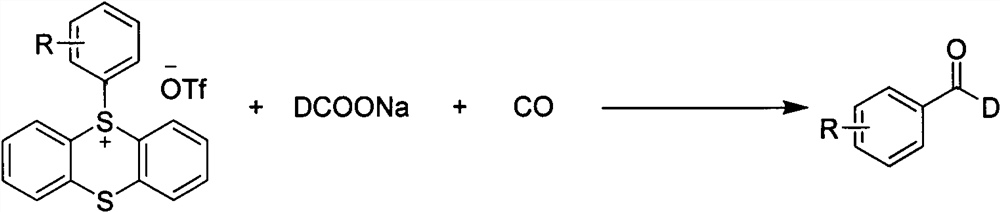
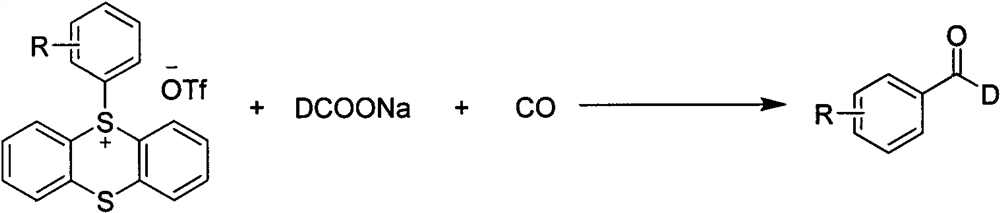
Synthesis method of palladium-catalyzed C-1 deuterated aromatic aldehyde
A C-1, aromatic aldehyde technology, applied in the field of palladium-catalyzed synthesis of C-1 deuterated aromatic aldehydes, can solve the problem that reaction conditions affect the scope of application of substrates, it is difficult to apply to practical applications in industrial production, and the deuterium selectivity is poor, etc. problem, to achieve the effect of good industrial application prospects, good adaptability and high reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] In a reaction tube with a stirrer, under the atmosphere of carbon monoxide, the catalyst PdCl was added in sequence 2 (PPh 3 ) 2 (0.02mmol, 10mol%), tris(1-naphthyl)phosphine as ligand (0.04mmol, 20mol%), aryl sulfate compound (0.2mmol, 1equiv), sodium deuterated formate (0.6mmol, 3equiv), tris Ethylamine (0.4mmol, 2equiv), the solvent is N,N-dimethylformamide solvent, and stirred at 120°C for 12h. After the reaction, the reaction mixture was filtered with celite, rinsed with ethyl acetate, the organic phases were combined, and the solvent was removed by a rotary evaporator to obtain a crude product. The crude product was separated by silica gel column chromatography using petroleum ether and ethyl acetate. The ester was used as the eluent to finally give the compound of formula 1 (83% isolated yield).
[0035]
[0036] 1 H NMR (400MHz, CDCl 3 )δ7.87-7.82(m, 2H), 7.02-6.99(m, 2H), 3.89(s, 3H); 13 CNMR (151MHz, CDCl 3 )δ190.6(t, J=26.5Hz), 164.6, 132.0, 129.9(t...
Embodiment 2
[0038] In a reaction tube with a stirrer, under the atmosphere of carbon monoxide, the catalyst PdCl was added in sequence 2 (PPh 3 ) 2 (0.02mmol, 10mol%), tris(1-naphthyl)phosphine as ligand (0.04mmol, 20mol%), aryl sulfate compound (0.2mmol, 1equiv), sodium deuterated formate (0.6mmol, 3equiv), tris Ethylamine (0.4mmol, 2equiv), the solvent is N,N-dimethylformamide solvent, and stirred at 120°C for 12h. After the reaction, the reaction mixture was filtered with celite, rinsed with ethyl acetate, the organic phases were combined, and the solvent was removed by a rotary evaporator to obtain a crude product. The crude product was separated by silica gel column chromatography using petroleum ether and ethyl acetate. The ester was used as the eluent to finally give the compound of formula 2 (isolated yield 64%).
[0039]
[0040] 1H NMR (600MHz, CDCl 3 )δ7.85-7.82(m, 2H), 7.53-7.51(m, 2H); 13 C NMR (151 MHz, CDCl 3 )δ190.6(t, J=26.9Hz), 141.0, 134.6(t, J=4.0Hz), 130.9, ...
Embodiment 3
[0042] In a reaction tube with a stirrer, under the atmosphere of carbon monoxide, the catalyst PdCl was added in sequence 2 (PPh 3 ) 2 (0.02mmol, 10mol%), tris(1-naphthyl)phosphine as ligand (0.04mmol, 20mol%), aryl sulfate compound (0.2mmol, 1equiv), sodium deuterated formate (0.6mmol, 3equiv), tris Ethylamine (0.4mmol, 2equiv), the solvent is N,N-dimethylformamide solvent, and stirred at 120°C for 12h. After the reaction, the reaction mixture was filtered with celite, rinsed with ethyl acetate, the organic phases were combined, and the solvent was removed by a rotary evaporator to obtain a crude product. The crude product was separated by silica gel column chromatography using petroleum ether and ethyl acetate. The ester was used as the eluent to finally give the compound of formula 3 (isolated yield 65%).
[0043]
[0044] 1 H NMR (400MHz, CDCl 3 )δ9.96(s, 0.01H), 7.82-7.79(m, 2H), 7.37-7.35(m, 2H), 2.61-2.55(m, 1H), 1.91-1.74(m, 5H), 1.49-1.35 (m, 4H), 1.30-1.24 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com