A kind of deuterated supramolecular polymer and preparation method thereof
A technology of supramolecular polymers and polymers, applied in the field of deuterium-substituted supramolecular polymers and its preparation, can solve the problems of complicated process and many preparation processes, and achieve the effects of simplifying the process, reducing side reactions, and simple sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
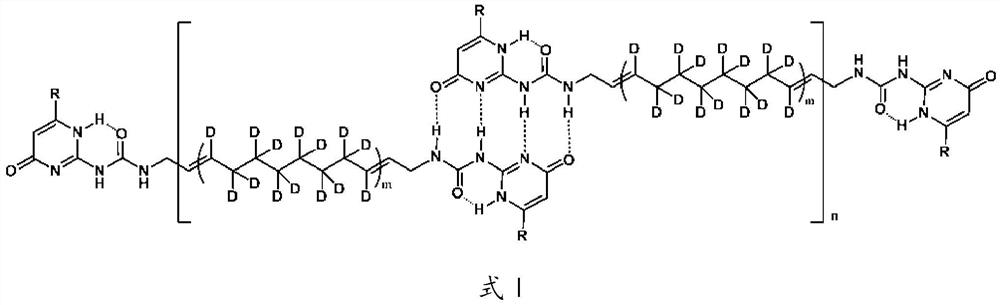
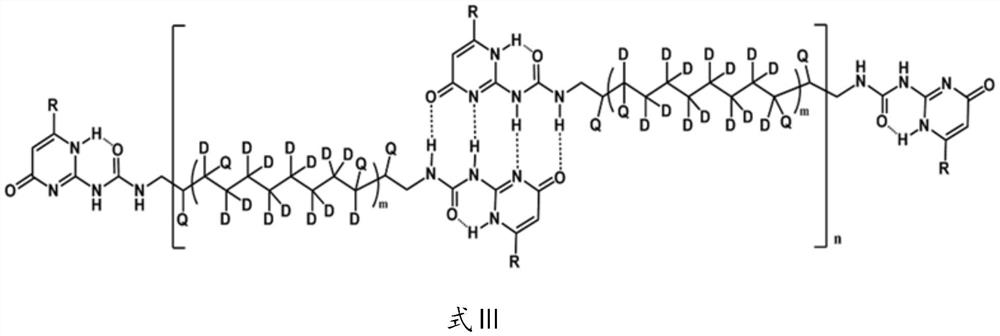
[0042] The specific preparation process of the quadruple hydrogen bond type deuterated supramolecular polymer of the present embodiment is as follows:
[0043] Take 42mL of non-deuterated cyclooctene, add 720mL of heavy water, 15.8g of RuCl 2 (PPh 3 ) 3 Catalyst, 5.8mL of ethanol, placed in a microwave reactor and heated at 180°C for 3h to obtain 30mL of deuterated cyclooctene with a deuterated rate of 95%; then take 200mmol of deuterated cyclooctene (22.8mL), add 4mmol of It is a chain transfer agent modified by 2-urea-4-pyrimidinone derivative (UPy), 20μmol Grubbs second-generation catalyst and 200mL dichloromethane, placed at 40°C for 10h, and then the solvent was evaporated to dryness under reduced pressure to obtain 22g quartet Hydrogen-bonded deuterated supramolecular polymers.
[0044] Take 10 g of the quadruple hydrogen bond type deuterated supramolecular polymer prepared above, dissolve it in 250 mL of dichloromethane, add 5% Pd / BaSO 4 Catalyst 2g, react at 60°C, 6M...
Embodiment 2
[0052] The specific preparation process of the quadruple hydrogen bond type deuterated supramolecular polymer of the present embodiment is as follows:
[0053] Take 42mL non-deuterated cyclopentene, add 454mL heavy water, 15.8g RuCl 2 (PPh 3 ) 3 Catalyst, 5.8mL of ethanol, placed in a microwave reactor and heated at 190°C for 2h to obtain 30mL of deuterated cyclopentene with a deuterated rate of 95%; then take 200mmol of deuterated cyclopentene (17.7mL), add 4mmol of It is a chain transfer agent modified by 2-urea-4-pyrimidinone derivative (UPy), 20μmol Grubbs second-generation catalyst and 50mL dichloromethane, react at 25°C for 20h, and then evaporate the solvent to dryness under reduced pressure to obtain 13g quadruple hydrogen bond type deuterated supramolecular polymers.
[0054] Take 10 g of the quadruple hydrogen bond type deuterated supramolecular polymer prepared above, dissolve it in 250 mL of dichloromethane, add 5% Pd / BaSO 4 Catalyst 2g, react at 60°C, 6MPa deu...
Embodiment 3
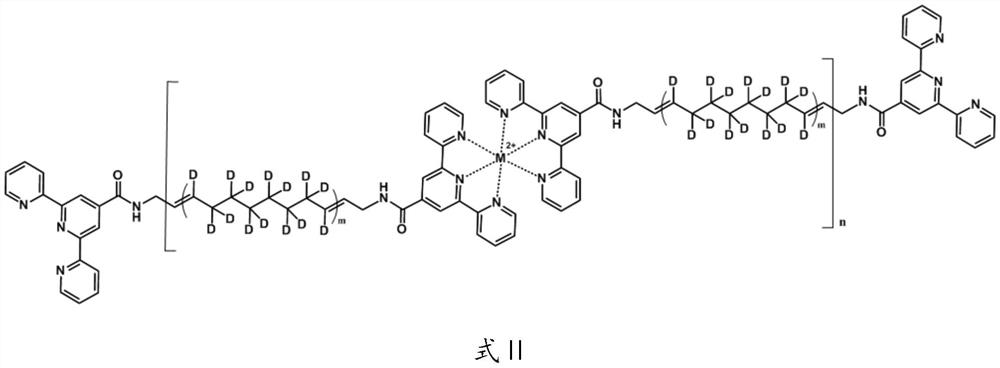
[0062] The specific preparation process of the metal coordination type deuterated supramolecular polymer in this embodiment is as follows:
[0063] Take 21 mL of non-deuterated cyclooctene, add 360 mL of heavy water, 7.9 g of RuCl 2 (PPh 3 ) 3 Catalyst, 2.9mL of ethanol, placed in a microwave reactor and heated at 190°C for 2h to obtain 15mL of deuterated cyclooctene with a deuterated rate of 95%; then take 100mmol of deuterated cyclooctene (12.4mL), add 2mmol of It is a chain transfer agent modified by terpyridine, 10 μmol of Grubbs third-generation catalyst and 160 mL of dichloromethane, placed at 40 ° C for 10 h, and then settled in 500 mL of methanol to obtain 11 g of deuterated cyclooctene as a spacer and triplets at both ends. Difunctional monomer of pyridine.
[0064] Get this difunctionality monomer 10g, add ZnPF 6 19mg, after dissolving in 400mL of dichloromethane, carry out supramolecular polymerization through the coordination of terpyridine and zinc ions, take...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com