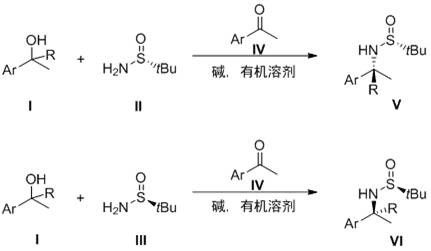
A kind of method of synthesizing chiral amine
A technology for chiral amines and compounds, applied in the field of synthesizing chiral amines, can solve the problems of low yield of deuterated products, expensive deuterated reagents, complicated reaction systems, etc., and achieves high reaction economic benefits, high atom utilization, The effect of simple reaction system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] In the glove box, 183 mg (1.5 mmol) of 1-phenylethanol, 60.5 mg (0.5 mmol) of R-(+)-tert-butylsulfinamide, 9 mg (0.075 mmol) of acetophenone, and 10 mg of sodium hydroxide ( 0.25 mmol) and 2 mL of toluene were added to the reaction tube, the reaction tube was sealed and taken out of the glove box, and the sealed tube was placed in a parallel reactor with a set temperature of 120 ° C and water condensed and refluxed for 12 h. After the reaction was finished, the diatomaceous earth was filtered to remove the alkali, the solvent was distilled off under reduced pressure, and column chromatography was separated to obtain a light yellow oily liquid with the following structural formula:
[0040]
[0041] Its yield is 72%, and the dr value measured by high performance liquid chromatography is 98.5:1.5. Its spectral data are: 1 H NMR (CDCl 3 ,400MHz)δ(ppm):7.36-7.27(m,1H),4.58-4.52(m,1H),3.41(brs,1H),1.51(d,J=6.4Hz,3H),1.24(s,9H ); 13 C NMR (CDCl 3 ,100MHz) δ (ppm): 144...
Embodiment 2
[0043] In the glove box, 302 mg (1.5 mmol) of 1-(4-bromophenyl) ethanol, 60.5 mg (0.5 mmol) of R-(+)-tert-butylsulfinamide, 15 mg (0.075 mmol) of 4-bromoacetophenone mmol), sodium hydroxide 10mg (0.25mmol), and toluene 2mL were added to the reaction tube, and the reaction tube was sealed and taken out of the glove box. . After the reaction was finished, diatomaceous earth was filtered to remove the alkali, the solvent was distilled off under reduced pressure, and column chromatography was separated to obtain a white solid with the following structural formula:
[0044]
[0045] Its yield is 78%, and the dr value measured by high performance liquid chromatography is 98.7:1.3. Its spectral data are: 1 H NMR (CDCl 3 ,400MHz)δ(ppm):7.47(d,J=8.4Hz,2H),7.23(d,J=8.4Hz,2H),4.53-4.48(m,1H),3.37(brd,J=2.0Hz, 1H), 1.49(d, J=6.4Hz, 3H), 1.23(s, 9H); 13 C NMR (CDCl 3 ,100MHz) δ (ppm): 143.1, 131.8, 128.4, 121.6, 55.6, 53.5, 22.6; HRMS (ESI) m / z theoretical value C 12 h 18 BrNOS[...
Embodiment 3
[0047] In the glove box, 228 mg (1.5 mmol) of 1-(4-methoxyphenyl) ethanol, 60.5 mg (0.5 mmol) of R-(+)-tert-butylsulfinamide, 4-methoxyphenyl ethyl Add 11.3mg (0.075mmol) of ketone, 10mg (0.25mmol) of sodium hydroxide, and 2mL of toluene into the reaction tube, seal the reaction tube and take it out of the glove box. The reactor was reacted for 12h. After the reaction was finished, the diatomaceous earth was filtered to remove the alkali, the solvent was distilled off under reduced pressure, and column chromatography was separated to obtain a light yellow oily liquid with the following structural formula:
[0048]
[0049] Its yield is 75%, and the dr value measured by high performance liquid chromatography is 98.5:1.5. Its spectral data are: 1 H NMR (CDCl 3 ,400MHz)δ(ppm):7.27(dt,J=8.8,2.4Hz,2H),6.88(dt,J=8.8,2.6Hz,2H),4.53-4.48(m,1H),3.8(s,1H ),3.33(brs,1H),1.48(d,J=6.4Hz,3H),1.22(s,9H); 13 C NMR (CDCl 3 ,100MHz) δ (ppm): 159.1, 136.2, 127.7, 114.0, 55.4, 55.2, 53.3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com