Dofetilide-d3 drug and preparation method thereof
A technology of -d3 and medicine, which is applied in the field of dofetilide-d3 medicine and its preparation, can solve the problems of easy introduction of by-products and toxicity, so as to avoid highly toxic reagents and high-risk reagents, reduce costs and reduce pollution and the effect of wasting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Embodiment 1: take p-chlorophenol as raw material, synthesize compound III
[0057]
[0058] 10.3 g of p-chlorophenol and 3.84 g of sodium hydroxide were respectively weighed into a 250 mL reaction flask, and 60 mL of ethanol was added. Then 44.8 g of 1,2-dibromoethane were slowly added dropwise at room temperature. After the dropwise addition was completed, the reaction was refluxed. After 24 hours, cool, spin dry the ethanol, and add water and ethyl acetate to the bottle. It was extracted three times with ethyl acetate, and the organic phases were combined and washed with water and saturated brine successively. The solvent was removed by rotary evaporation, and then the pure target product was obtained by column chromatography (developing solvent: petroleum ether / ethyl acetate) in a yield of 51%.
Embodiment 2
[0059] Example 2: Synthesis of Compound I
[0060]
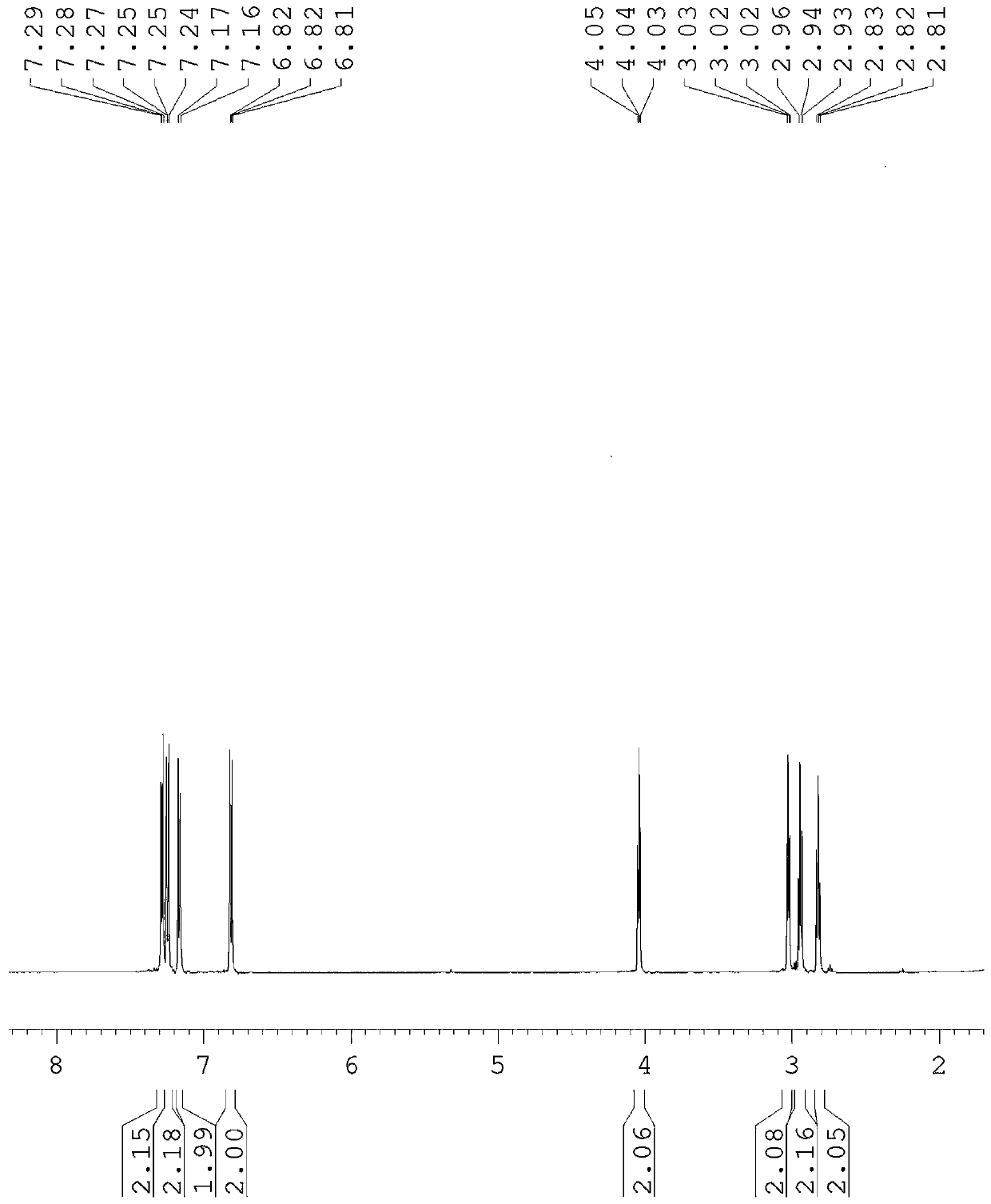
[0061] 3.5 g of compound III, 3.5 g of p-chlorophenethylamine and 4.14 g of potassium carbonate were respectively weighed, and 15 mL of anhydrous acetonitrile was added. Then react at 80°C. After 24 hours, the solvent was directly evaporated by rotary evaporation, and then the pure target product was obtained by column chromatography (developing solvent: dichloromethane / methanol), such as figure 1 shown, by 1 The structure was confirmed by HNMR and other tests, and the yield was 91%.
Embodiment 3
[0062] Example 3: Synthesis of Compound II
[0063]
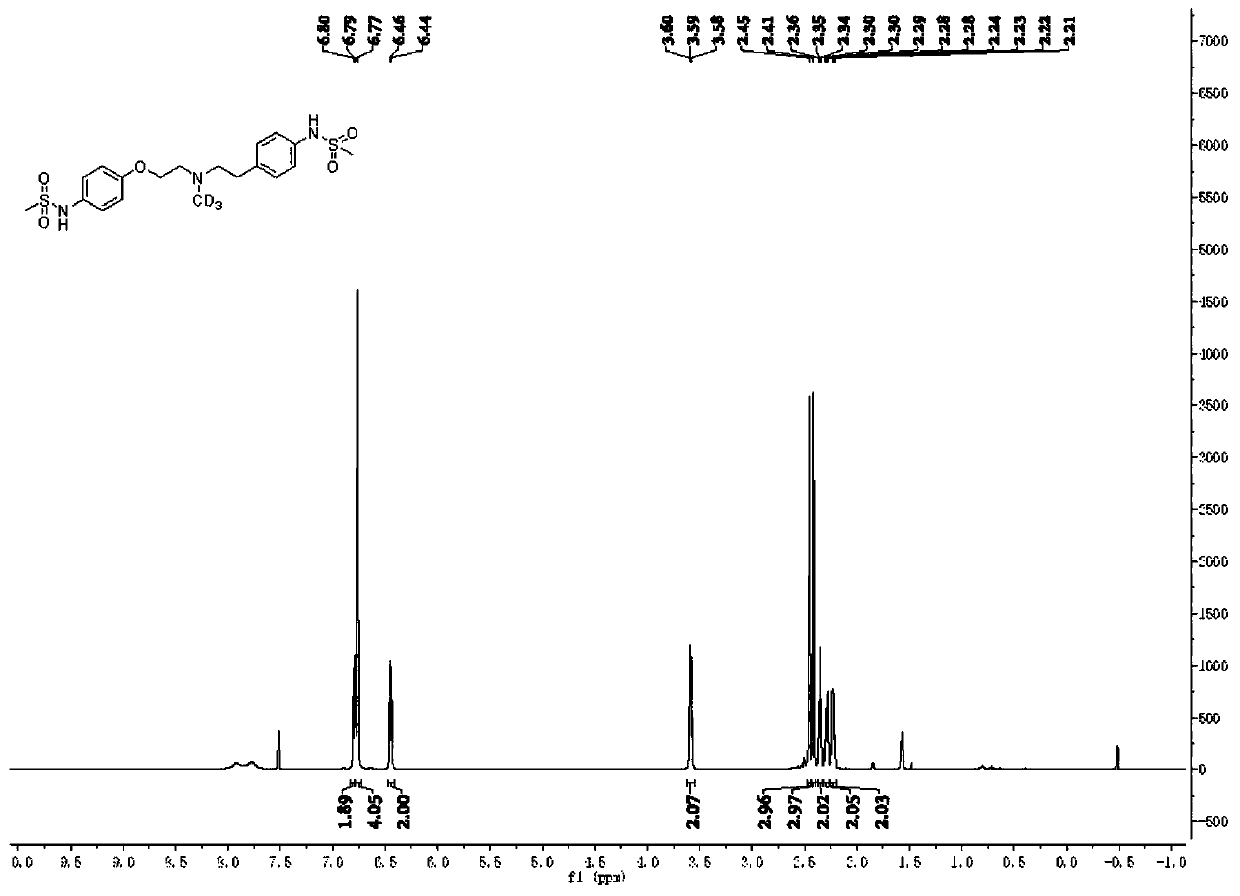
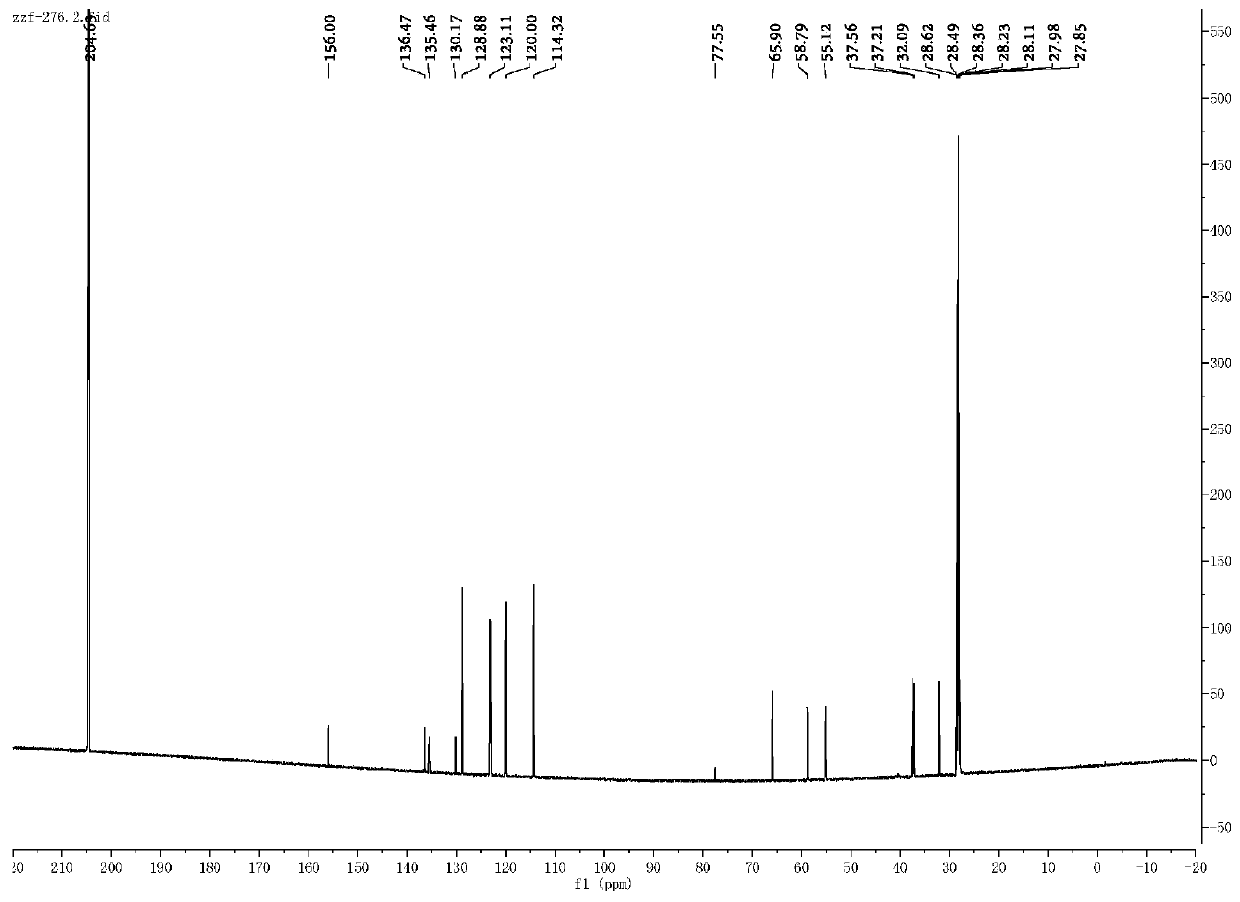
[0064] Weigh 0.4 mmol of compound I, 25.0 mg of Pd / KPCN photocatalyst and 40 mg of aluminum trichloride into a 5.0 mL reaction flask, add 2.0 mL of anhydrous acetonitrile, and add deuterium water / d 4 - a mixed solution of deuterated methanol (1.0mL / 0.6mL), replace the reaction system with an argon protection state, then place the reaction flask under a 420nm light source for 24 hours of light reaction, remove the light source after the reaction, and place the reaction mixture pad Celite filter, then with 5.0mL CH 2 Cl 2 After extraction, the extract was dried over anhydrous sodium sulfate and concentrated to obtain a colorless liquid. The solvent was removed by rotary evaporation, and then the pure target product was obtained by column chromatography (developing solvent: dichloromethane / methanol). 1 The structure was confirmed by HNMR, C-NMR and other tests, the yield was 96%, and the deuteration rate was >95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com