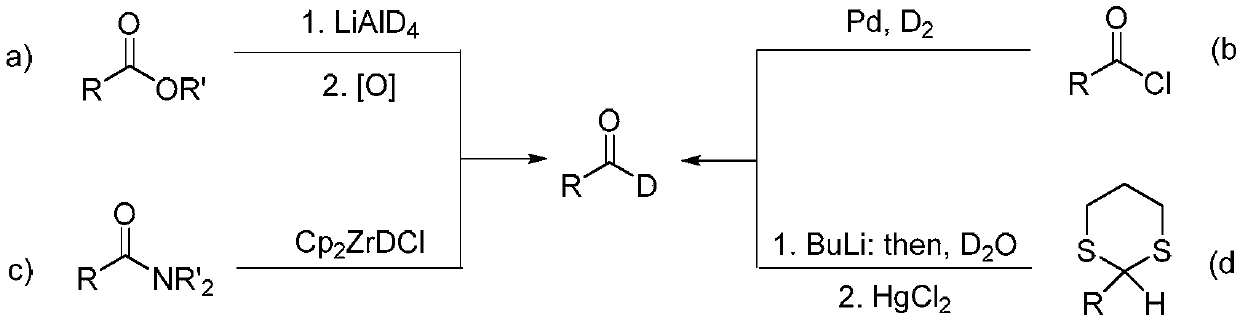
Method for preparing deuterated aldehyde through triazole carbene catalysis
A technology of triazole carbene and aldehyde substitution, which is applied in the field of triazole carbene to catalyze the preparation of deuterated aldehydes, which can solve the problems of reduced atom economy, low substrate applicability, and high reaction by-products, achieving high economy and applicability , wide range of substrate applicability, and the effect of reducing reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
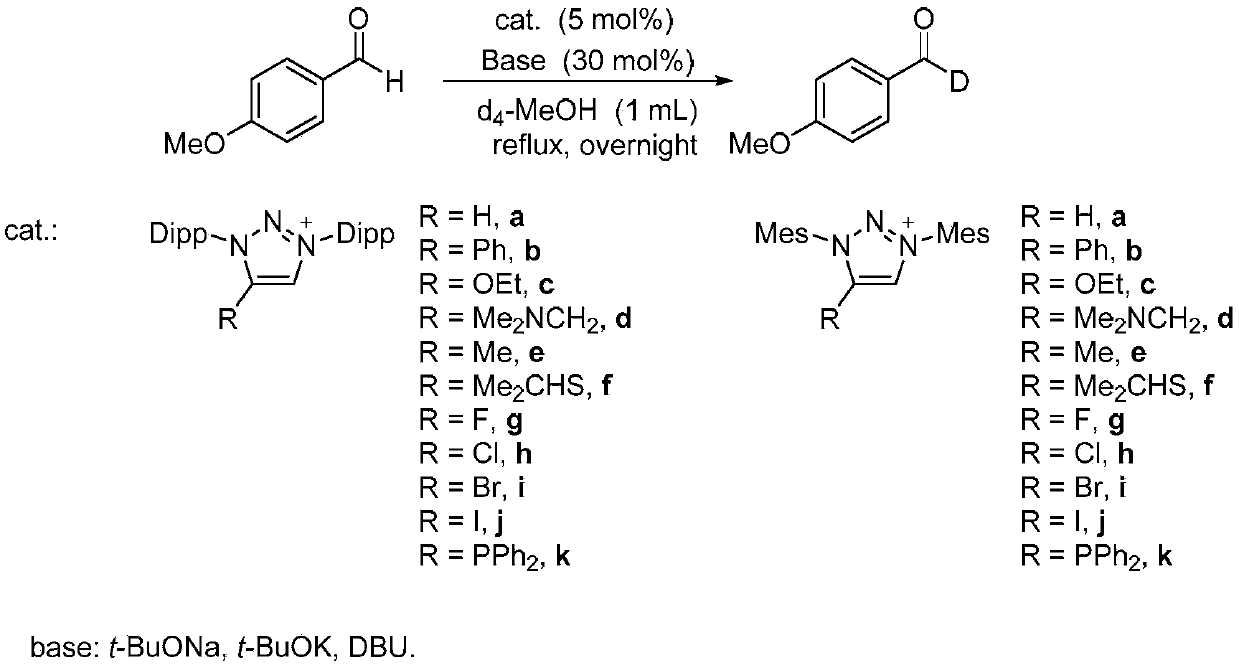
[0048] In the glove box, p-methoxybenzaldehyde (0.2 mmol), triazole carbene salt (0.01 mmol, R=PPh 2 ), potassium tert-butyl alkoxide (0.06mmol) and deuterated methanol (1mL) were reacted under reflux for 12 hours. The reaction solution was cooled, the solvent was spin-dried, and column chromatography was performed on silica gel to obtain the target product, 22.6 mg of a yellow oil, with a yield of 82%.
[0049] Structural characterization of the product.
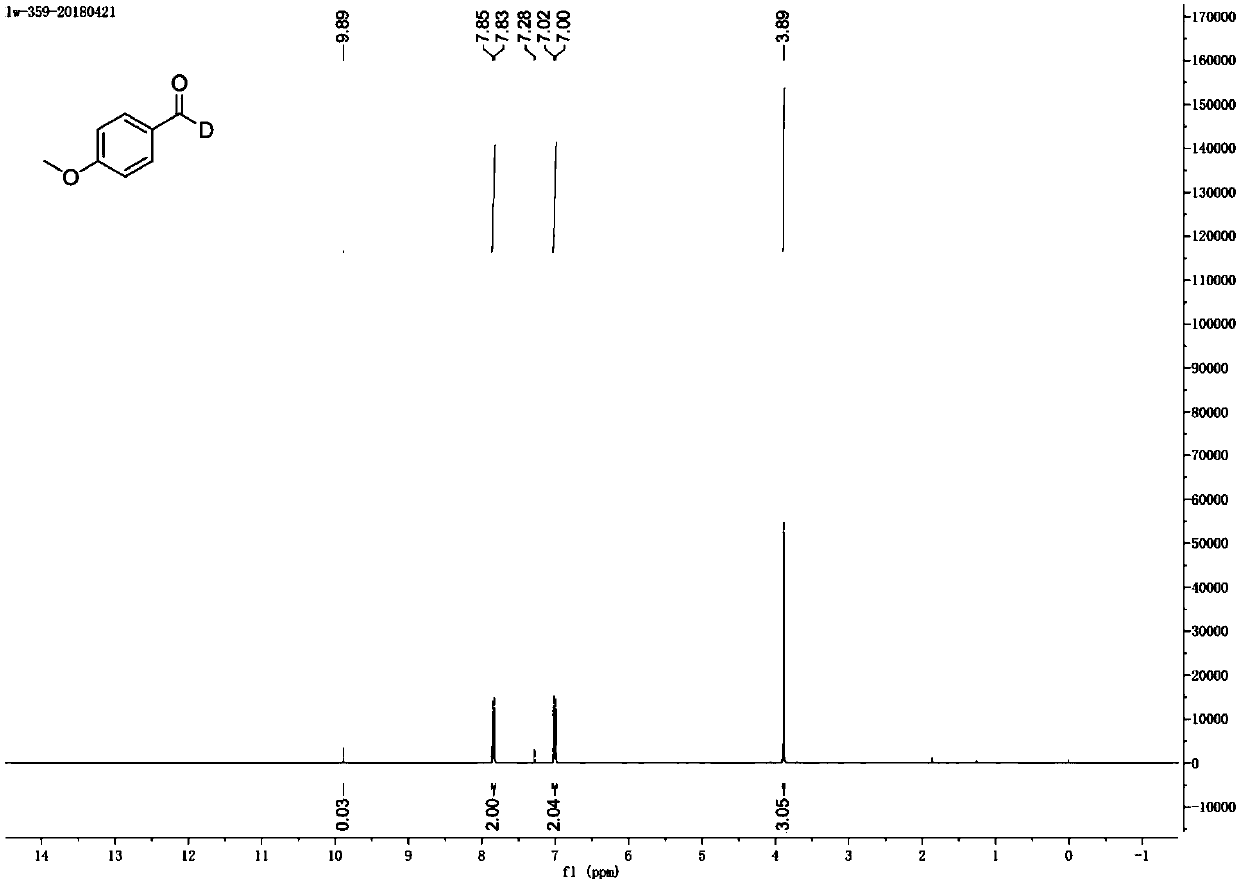
[0050] image 3 for the product 1 H NMR spectrum.
[0051] Figure 4 for the product 13 C NMR spectrum.
[0052] Characterization data for the deuterated compound only: 1 H NMR (400MHz, CDCl 3 )δ7.84(d, J=8.8Hz, 2H), 7.01(d, J=8.8Hz, 2H), 3.89(s, 3H). 13 C NMR (100MHz, CDCl 3 )δ190.5 (t, J=26.2Hz), 164.6, 132.0, 129.9, 114.3, 55.6. HRMS (ESI-TOF) m / z CalcdforC 8 h 7 do 2 [M+H] + 138.0665,found 138.0658.
[0053] Thus it can be known that the product obtained is indeed deuterated p-methoxybenzaldehyde.
Embodiment 2
[0055] In the glove box, 2-naphthaldehyde (0.2 mmol), triazole carbene salt (0.01 mmol, R=PPh 2 ), potassium tert-butyl alkoxide (0.06mmol) and deuterated methanol (1mL) were reacted under reflux for 12 hours. The reaction solution was cooled, the solvent was spin-dried, and column chromatography was performed on silica gel to obtain the target product, 23.4 mg of a white solid, and the yield: 75%.
[0056] Figure 5 for the resulting product 1 H NMR spectrum.
[0057] Figure 6 for the product 13 C NMR spectrum.
[0058] Characterization data for the deuterated compound only: 1 H NMR (400MHz, CDCl 3 )δ8.36(s,1H),8.03(d,J=8.0Hz,1H),8.01–7.95(m,2H),7.93(d,J=8.9Hz,1H),7.73–7.64(m,1H ),7.64–7.59(m,1H). 13 C NMR (100MHz, CDCl 3 )δ191.9 (t, J=26.7Hz), 136.5, 134.5, 134.1, 132.7, 129.5, 129.1, 129.1, 128.1, 127.1, 122.8. HRMS (ESI-TOF) m / zCalcd for C 11 h 7 DO[M+H] + 158.0711, found 158.0707.
[0059] Thus it can be known that the resulting product is indeed deuterated...
Embodiment 3
[0061] In the glove box, add 3-(4-isopropylphenyl)-2-methylpropanal (0.2mmol), triazole carbene salt (0.01mmol, R=PPh 2 ), potassium tert-butyl alkoxide (0.06mmol) and deuterated methanol (10mL), reacted under reflux conditions for 12 hours. The reaction solution was cooled, the solvent was spin-dried, and column chromatography was performed on silica gel to obtain the target product, 37.6 mg of a colorless liquid, with a yield of 99%.
[0062] Figure 7 for the resulting product 1 H NMR spectrum.
[0063] Figure 8 for the product 13 C NMR spectrum.
[0064] Characterization data for the deuterated compound only: 1 H NMR (400MHz, CDCl 3 )δ7.16(d, J=8.1Hz, 2H), 7.09(d, J=8.1Hz, 2H), 3.04(d, J=14.0Hz, 1H), 2.88(hept, J=6.9Hz, 1H) ,2.58(d,J=14.0Hz,1H),1.24(d,J=6.9Hz,6H),1.08(s,3H). 13 C NMR (100MHz, CDCl 3 )δ204.4(t, J=26.0Hz), 147.0, 136.1, 128.9, 126.6, 47.5(t, J=16.2Hz), 36.2, 33.7, 24.0, 13.12.HRMS(ESI-TOF)m / z Calcd for C 13 h 16 D. 2 O[M+Na] + 193.1556,found ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com