A kind of method that takes halomethyl compound as raw material to prepare deuterated aldehyde
A compound, halomethyl technology, applied in the field of compound preparation, can solve problems such as harsh reaction conditions, limited substrate range, cumbersome multi-step operation, etc., and achieve the effect of simple reaction conditions, simple operation and wide applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
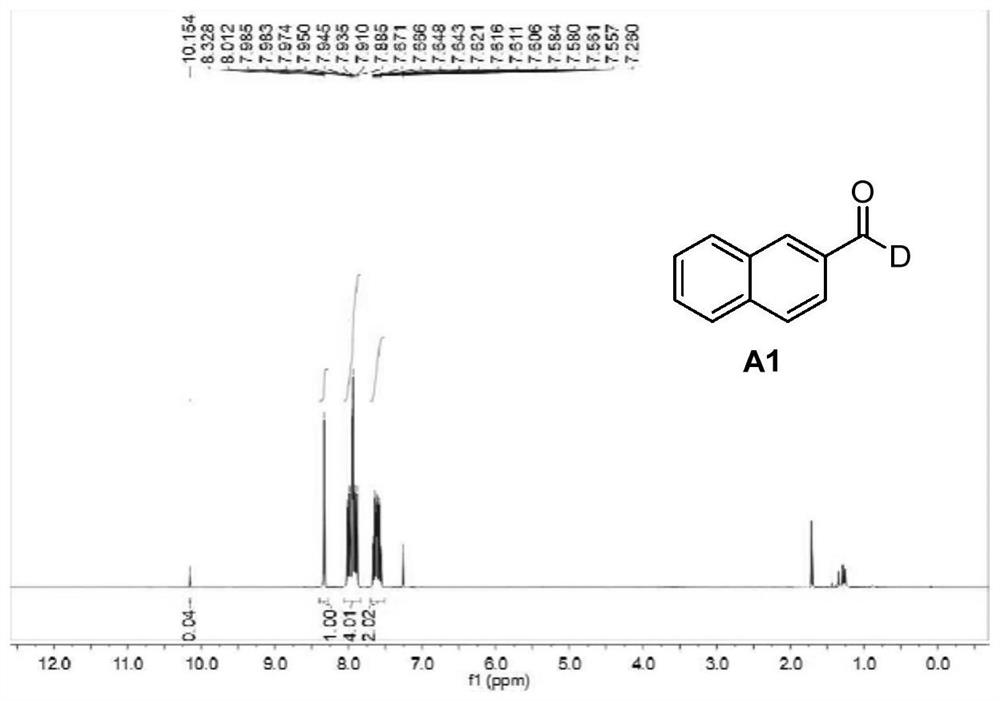
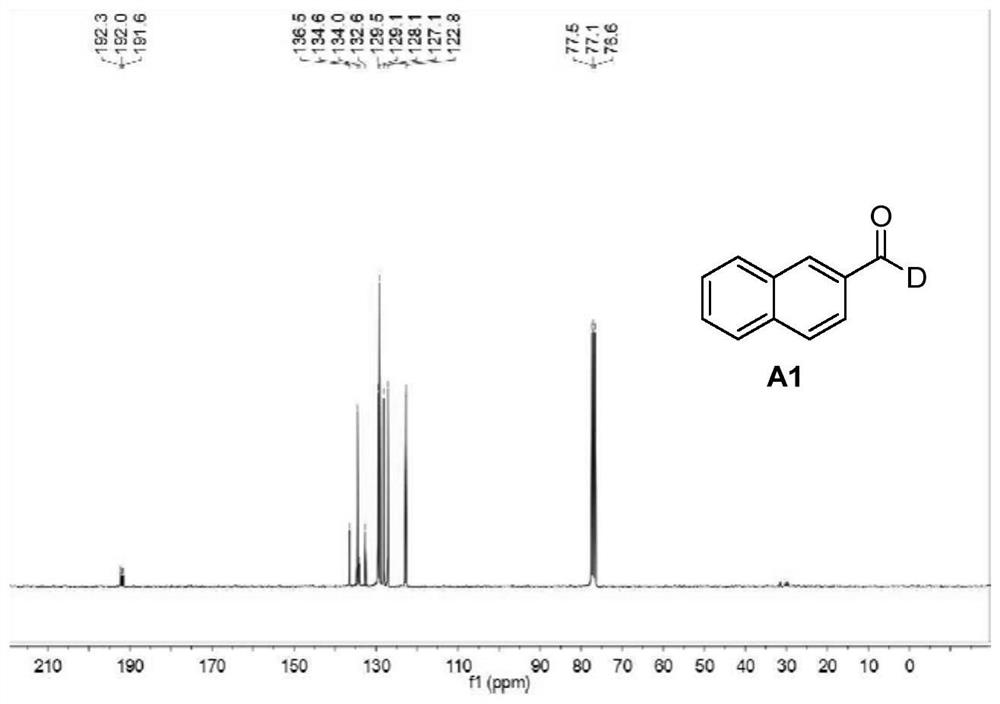
[0044] Embodiment 1: the preparation of 2-deuterated naphthaldehyde (A1)
[0045] First mix DMSO (3mL) with D 2 O (1 mL) was mixed and stirred for 5 minutes, then 2-naphthylmethyl bromide (0.5 mmol), 4-picoline (2 mmol), potassium phosphate (2 mmol) were added sequentially. The reaction was stirred at room temperature for 10 hours, then 4-dimethylaminonitrosobenzene (1 mmol) was added at room temperature. The mixed reaction solution was continued to react at room temperature for 4 hours, then slowly added dropwise 12 mL of 3M hydrochloric acid solution under ice-bath conditions, then continued to stir for 1 hour, then stopped the reaction and carried out post-treatment using the following method: The reaction solution was extracted three times and the organic layers were combined, and the organic layer was washed twice with water and saturated brine. Then add anhydrous sodium sulfate for drying, and finally perform column chromatography through a silica gel column (DCM:MeOH=...
Embodiment 2
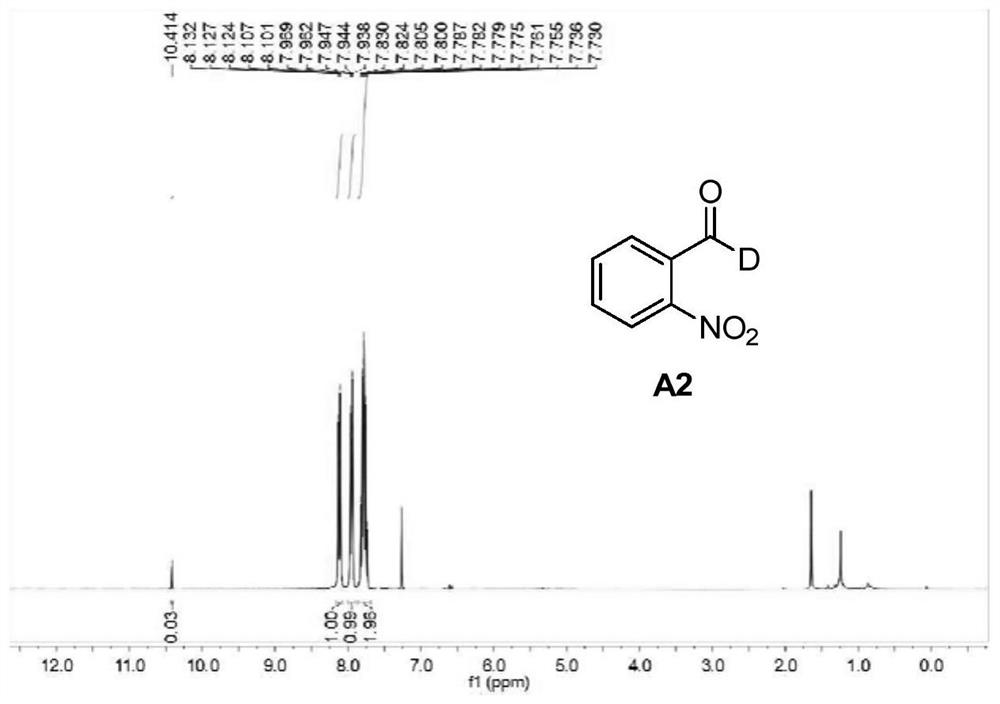
[0047] Embodiment 2: the preparation of A2
[0048] Except that 2-nitrobenzyl bromide is used to replace 2-naphthylmethyl bromide in Example 1, and the reaction temperature is 50° C., other conditions and steps are the same as in Example 1 to obtain Compound A2 (56% yield, 97% deuteration rate). %).
[0049] Pale yellow solid. 1 H NMR (300MHz, CDCl 3 ):δ10.41(s,0.03H),8.10-8.13(m,1H),7.94-7.97(m,1H),7.30-7.83(m,2H). 13 C NMR (75MHz, CDCl 3 ):δ187.8(t,J C-D =29.2Hz), 149.6, 134.1, 133.7, 131.3, 129.7, 124.5.
Embodiment 3
[0050] Embodiment 3: the preparation of A3
[0051] Except that 4-cyanobenzyl bromide was used instead of 2-naphthylmethyl bromide in Example 1, other conditions and steps were the same as in Example 1 to obtain compound A3 (yield 84%, deuteration rate 97%).
[0052] white solid. 1 H NMR (600MHz, CDCl 3 ):δ10.09(s,0.03H),7.98(d,2H,J=8.4Hz),7.83(d,2H,J=8.4Hz). 13 C NMR (150MHz, CDCl 3 ):δ189.8(t,J C-D =27.0Hz), 138.2, 132.4, 129.4, 117.2, 117.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com