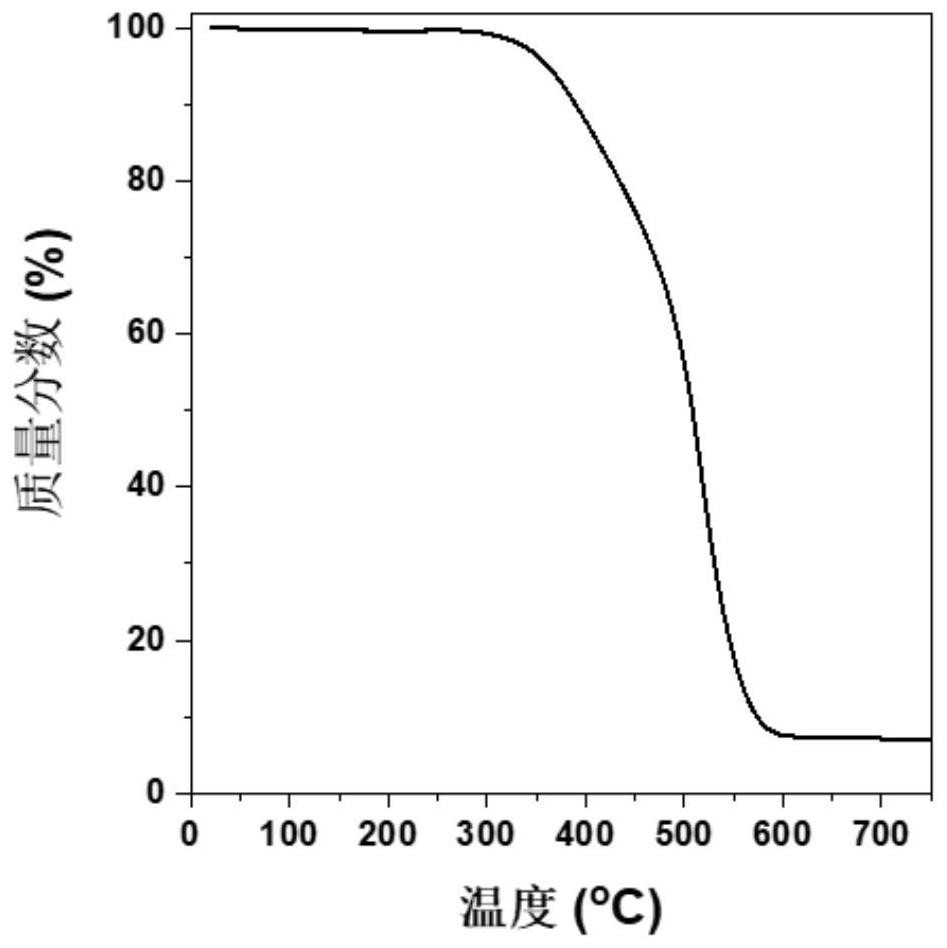
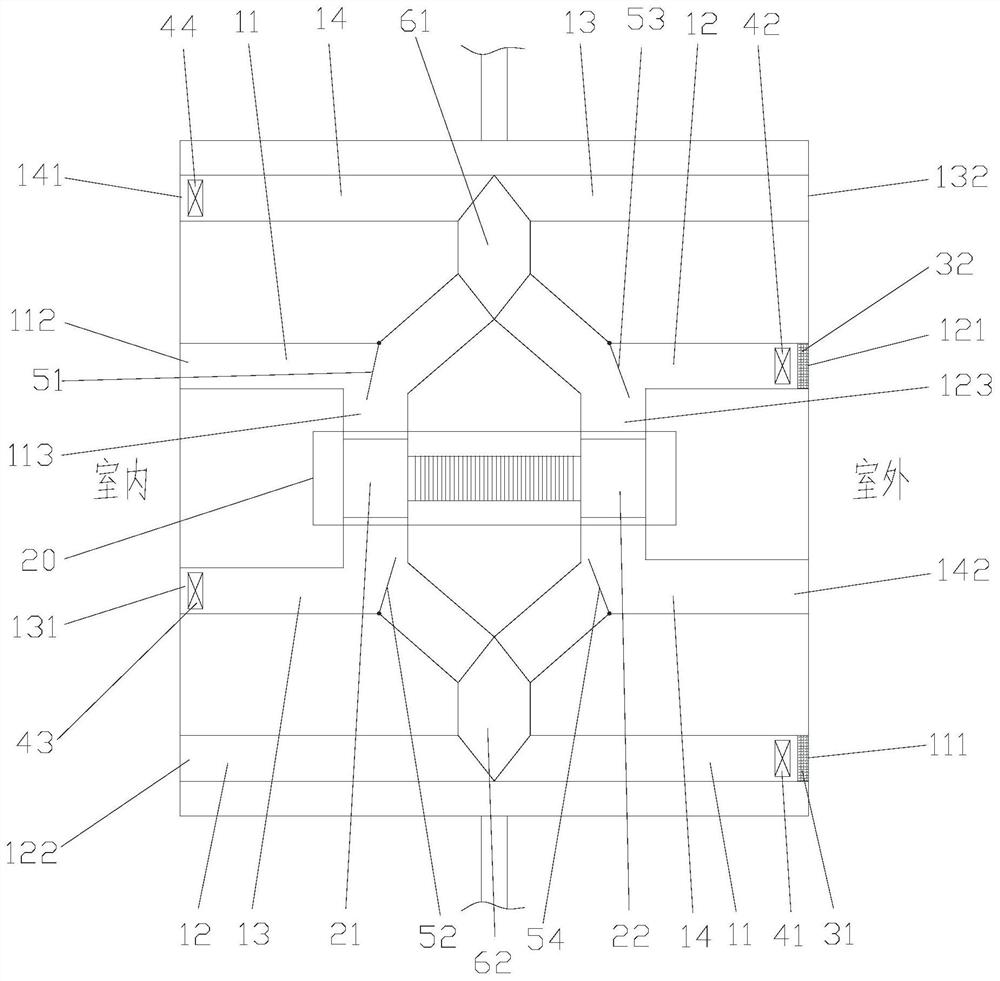
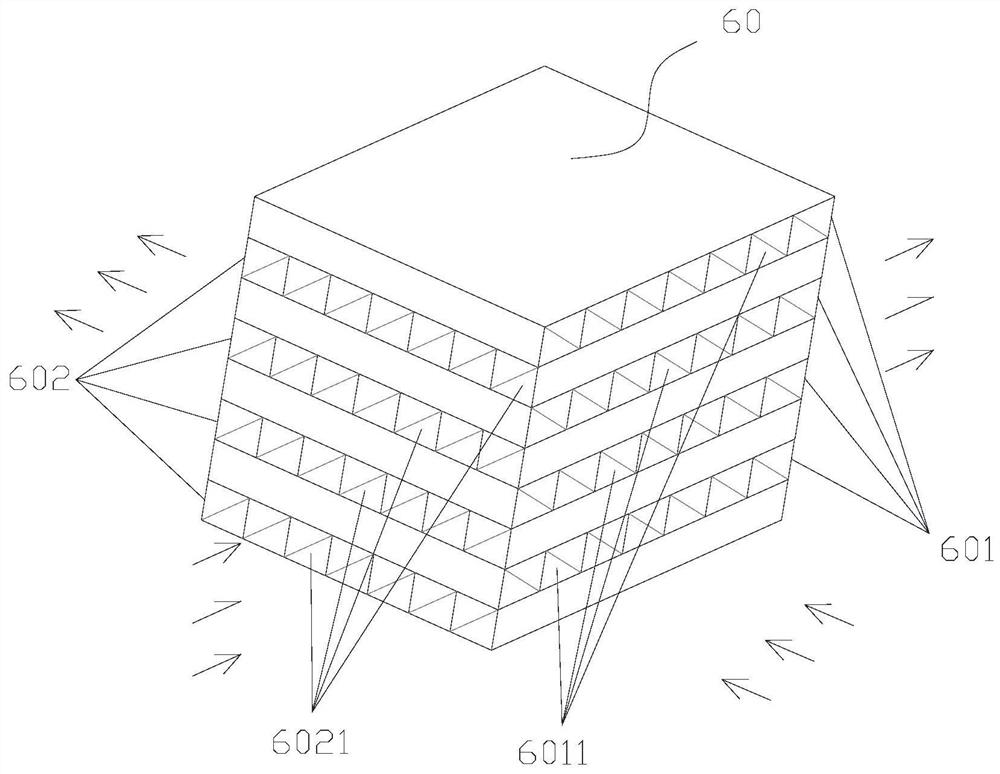
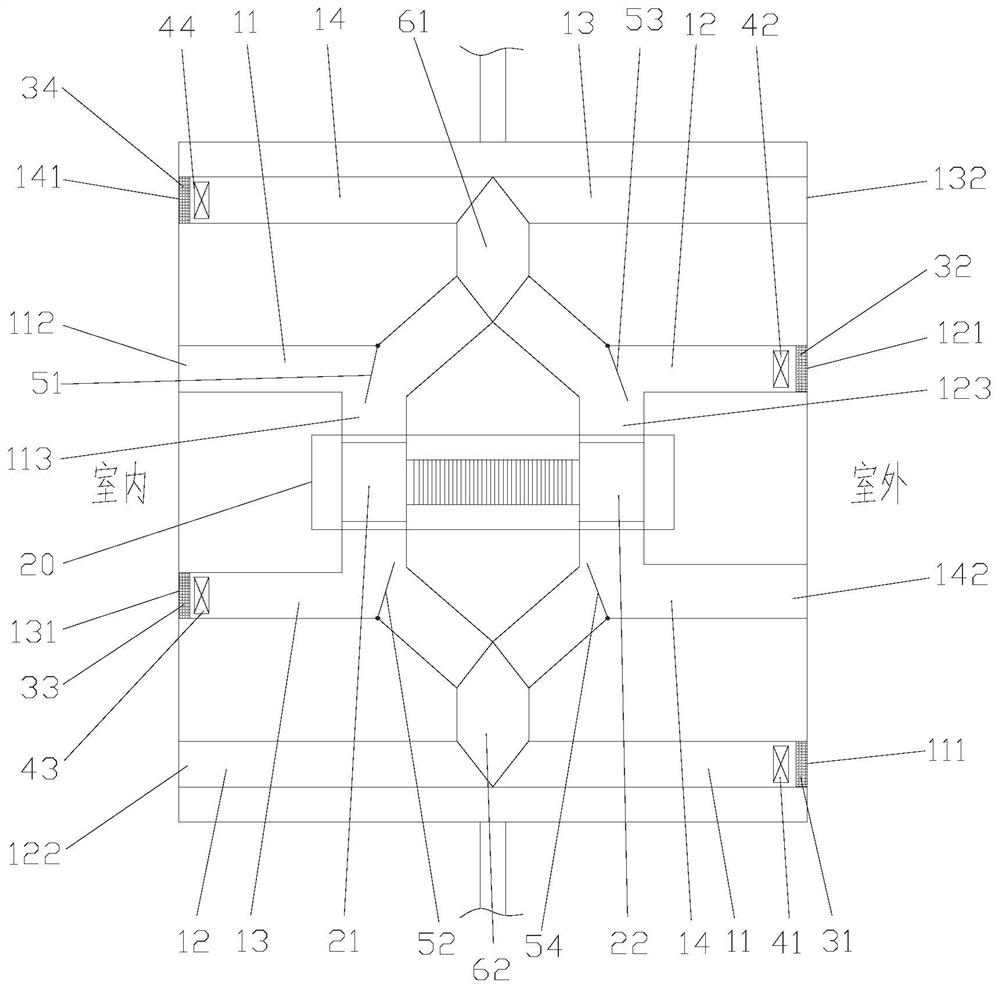
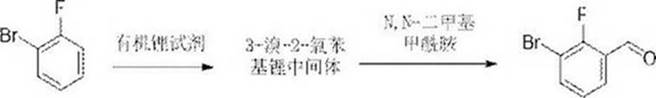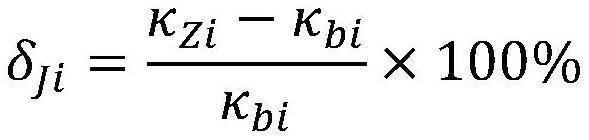Patents
Literature
35 results about "Hydrogen exchange" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
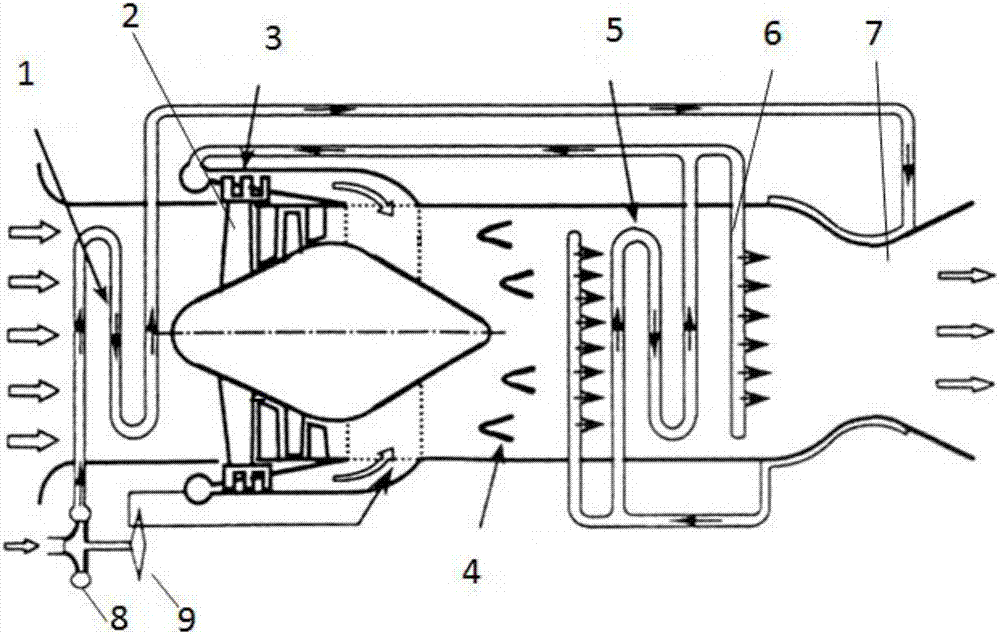
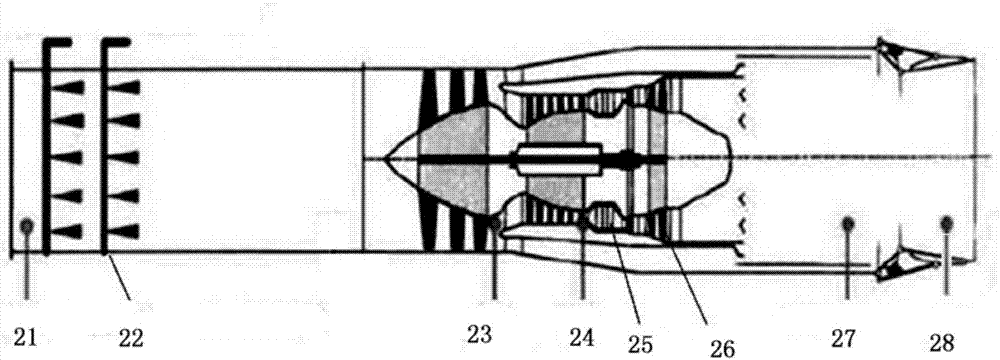
Air precooling compression aircraft engine and hypersonic velocity aircraft
ActiveCN106014637AHigh fuel specific impulseImprove economyTurbine/propulsion engine coolingGas turbine plantsCombustion chamberHydrogen exchange
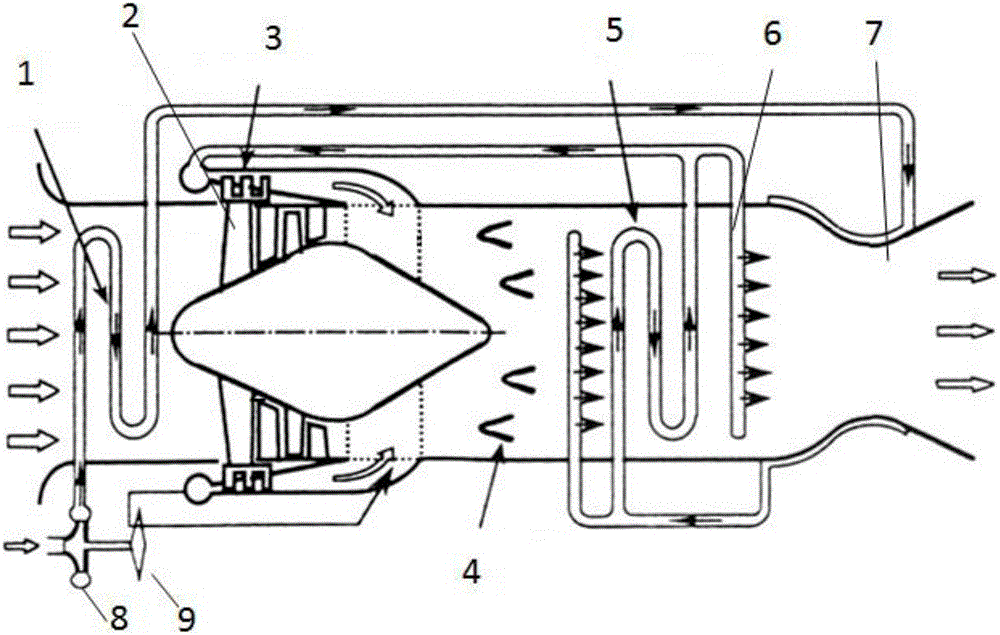
The invention discloses an air precooling compression aircraft engine and a hypersonic velocity aircraft. The air precooling compression aircraft engine comprises an intake way, an air compressor, a combustion chamber and a spraying pipe arranged in sequence; and the air compressor is provided with a turbine for providing driving force. The air precooling compression aircraft engine further comprises a first heat exchanger for adopting a circular cooling agent to cool air introduced by the intake way, a cooling agent pump provided with a cooling agent outlet communicated with the first heat exchanger and a cooling agent recovery port communicated with a second heat exchanger for recovering the cooling agent, the second heat exchanger for using liquid hydrogen output by a liquid hydrogen pump as a cold source to cool the heated cooling agent and leading out the cooled cooling agent to the cooling agent pump for recycling, the liquid hydrogen pump connected with an inlet of the second heat exchanger for supplying liquid hydrogen as a fuel, and an injector positioned between the air compressor and the combustion chamber and communicated with the second heat exchanger for injecting air compressed by the air compressor and hydrogen exchanged heat by the second heat exchanger into the combustion chamber.
Owner:NAT UNIV OF DEFENSE TECH
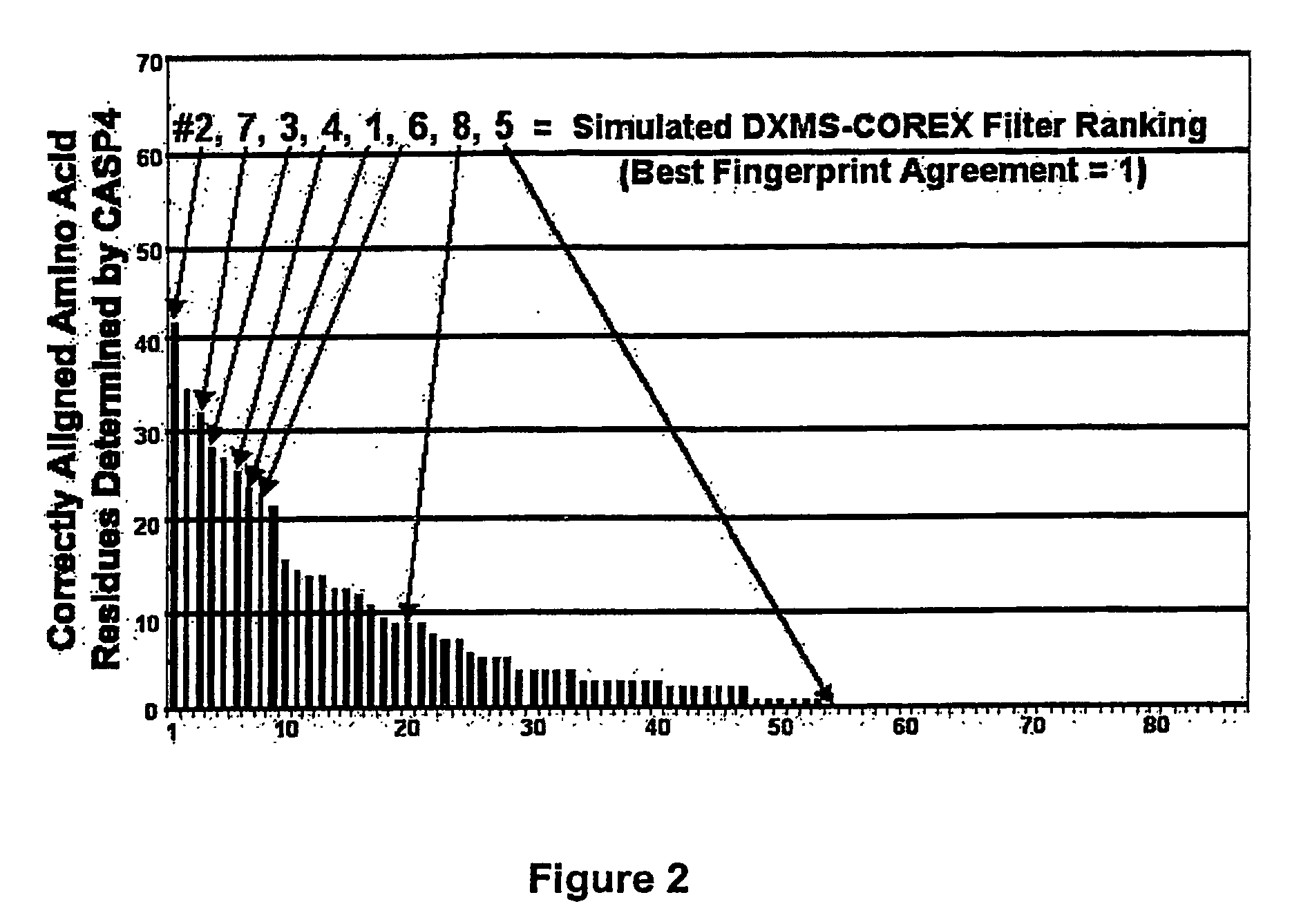
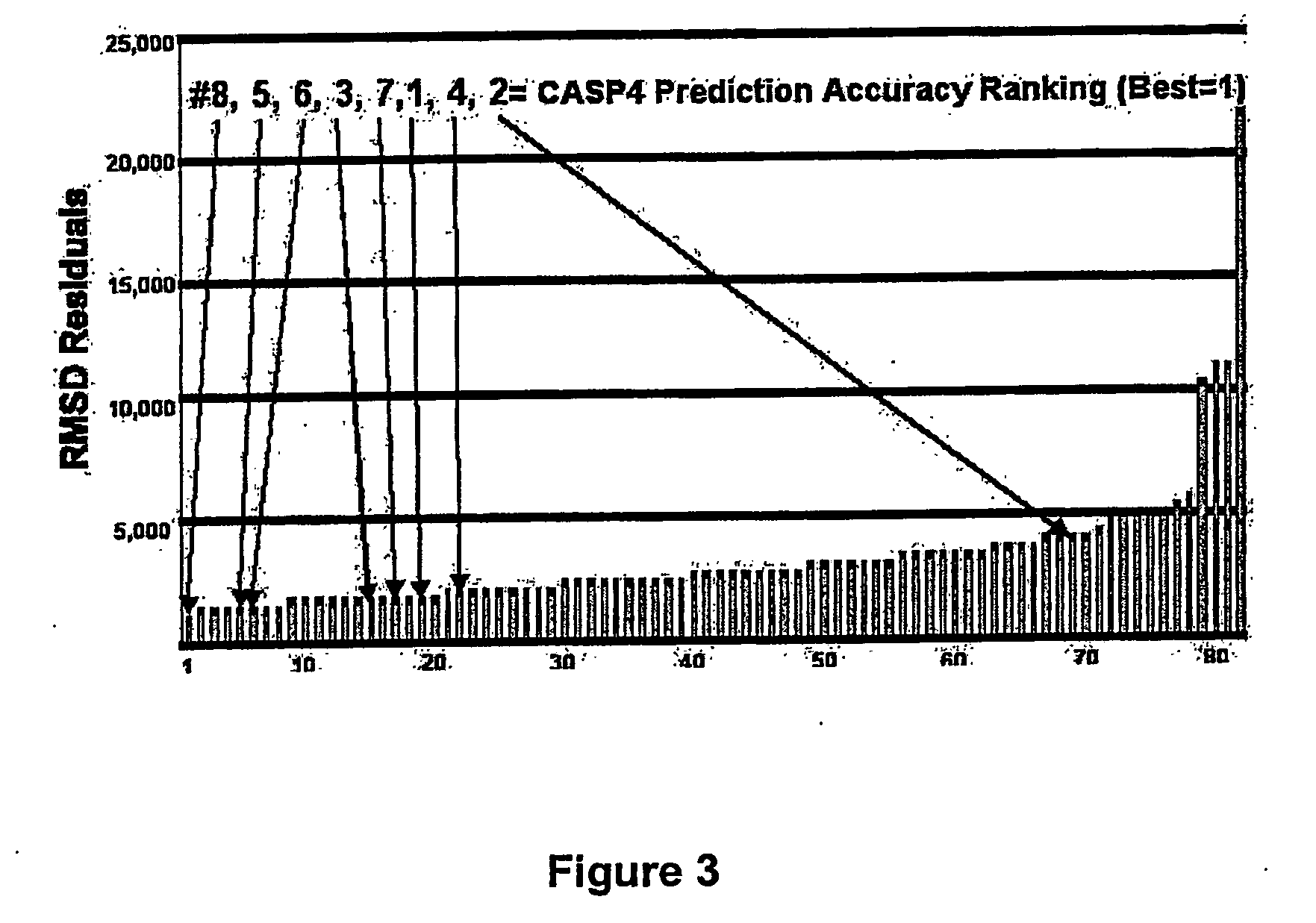
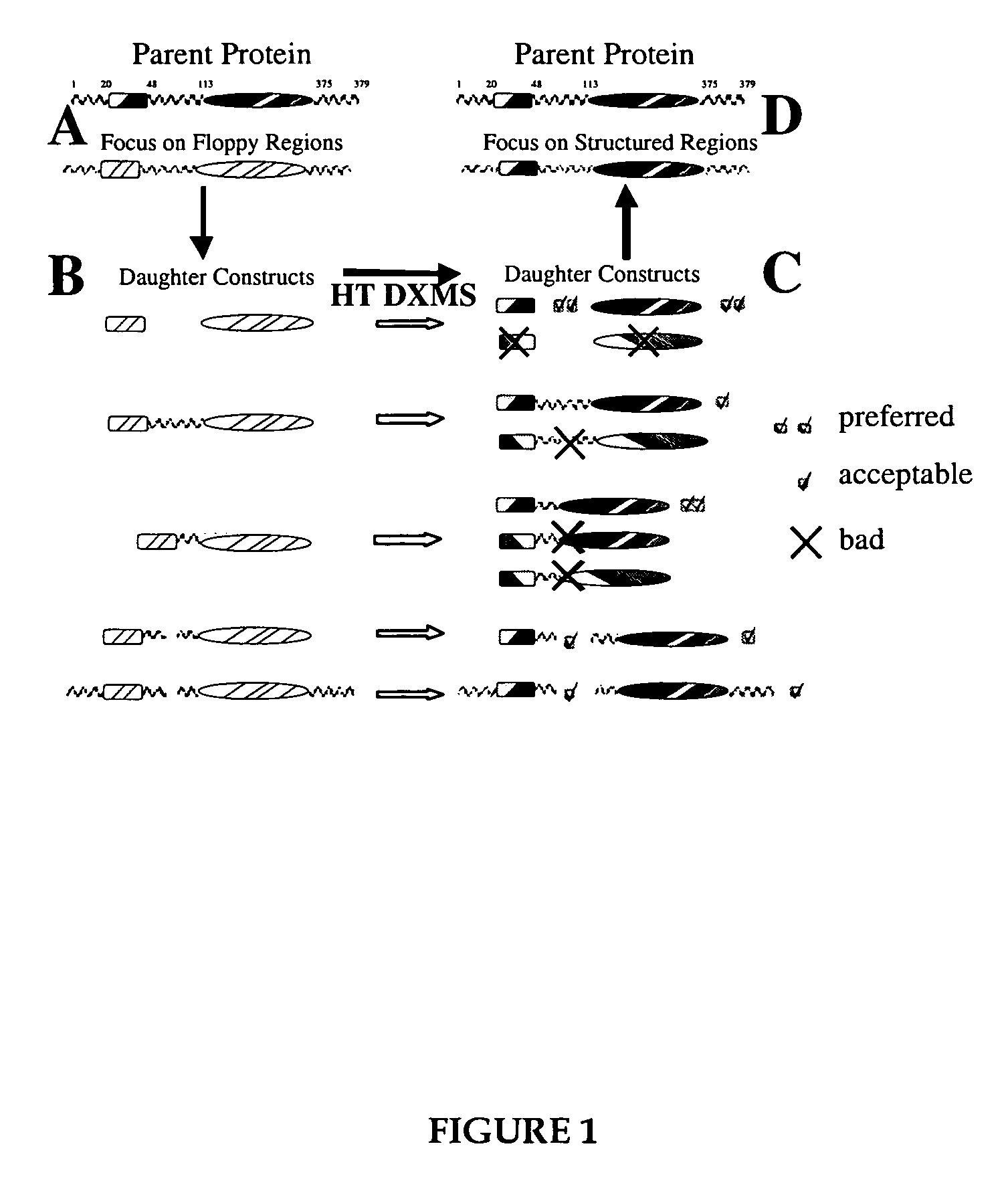
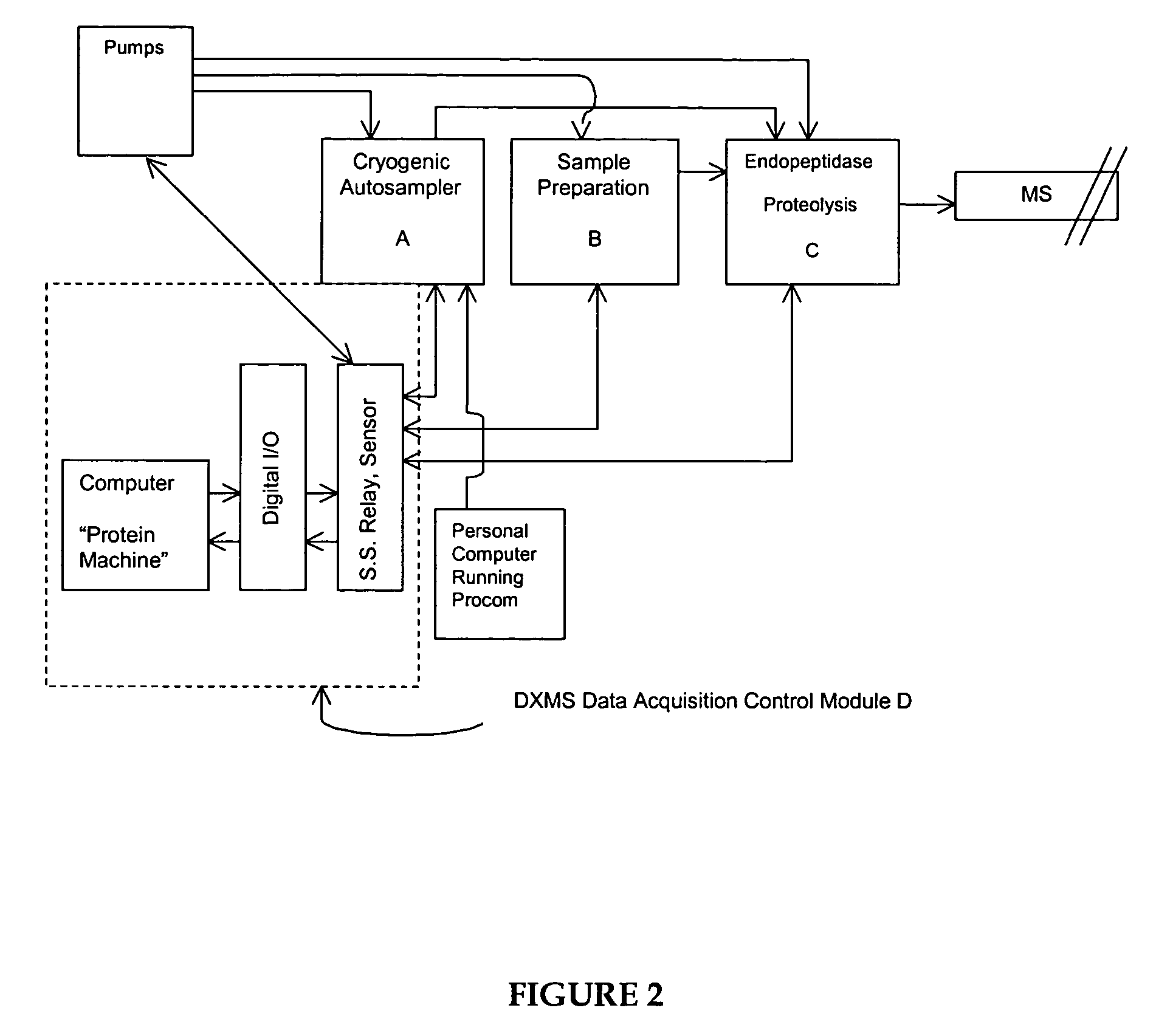
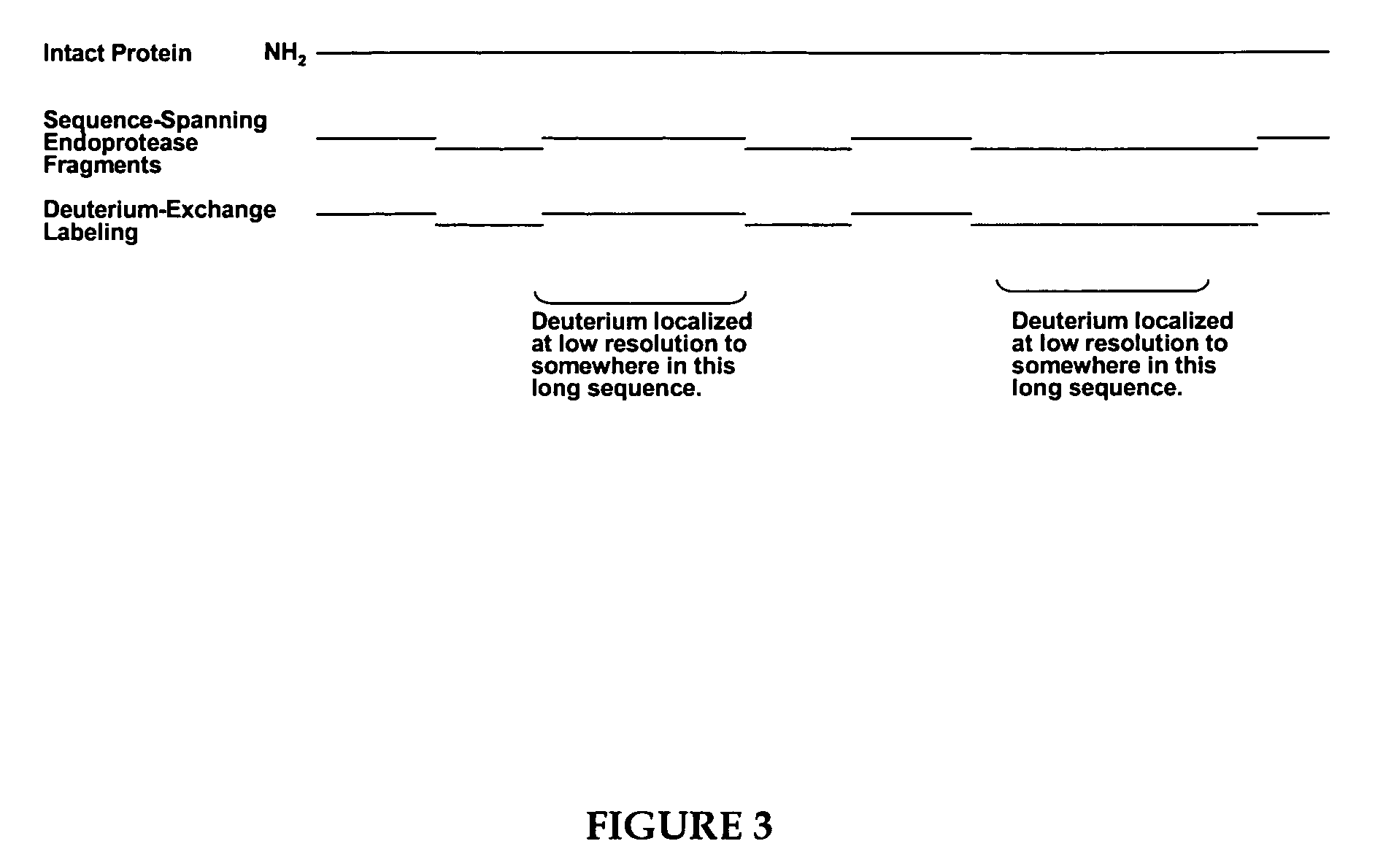
Methods for the determination of protein three-dimensional structure employing hydrogen exchange analysis to refine computational structure prediction
InactiveUS20070122864A1Improve accuracySpeeding calculationMicrobiological testing/measurementBiological material analysisCrystallographyHydrogen exchange
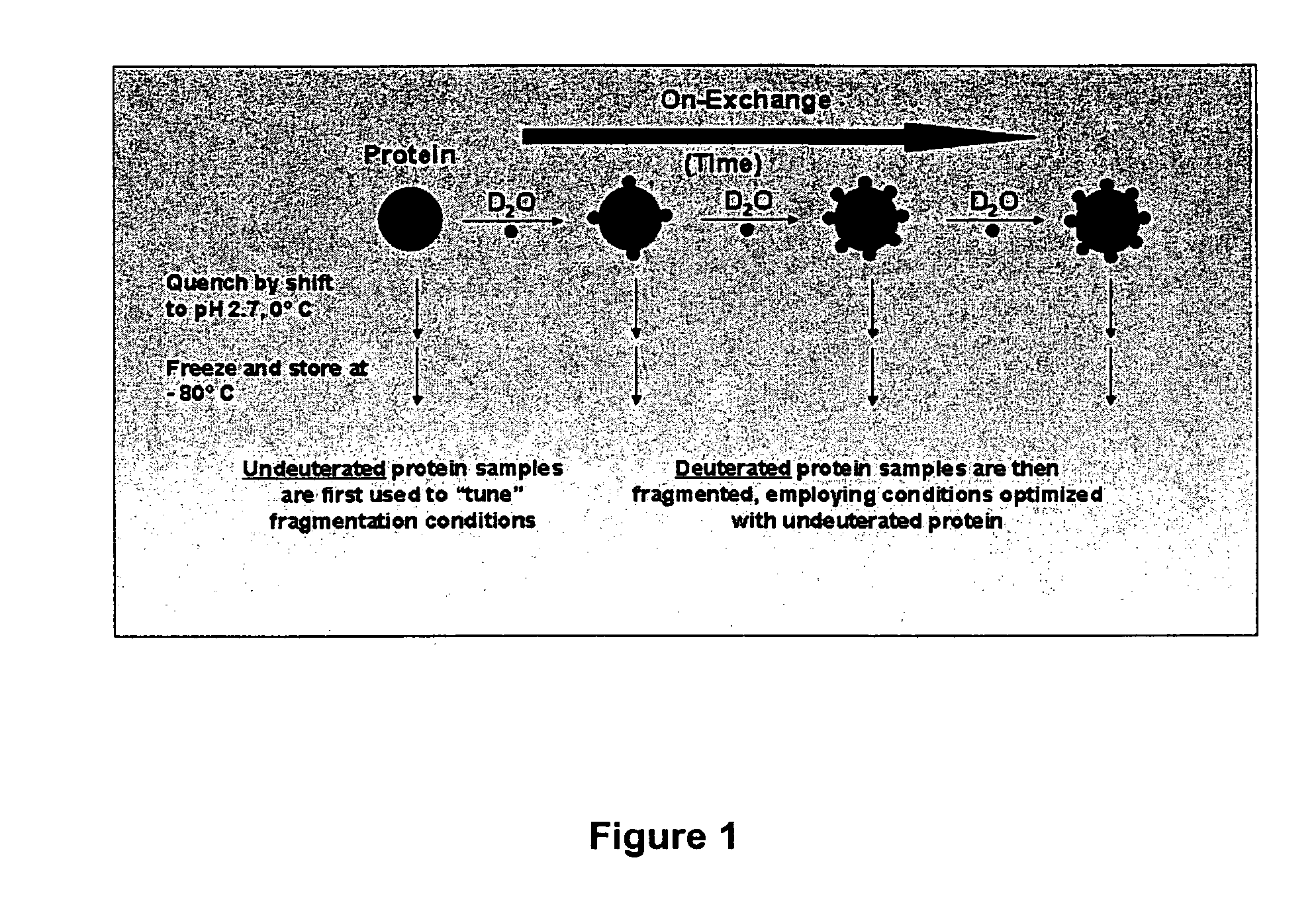
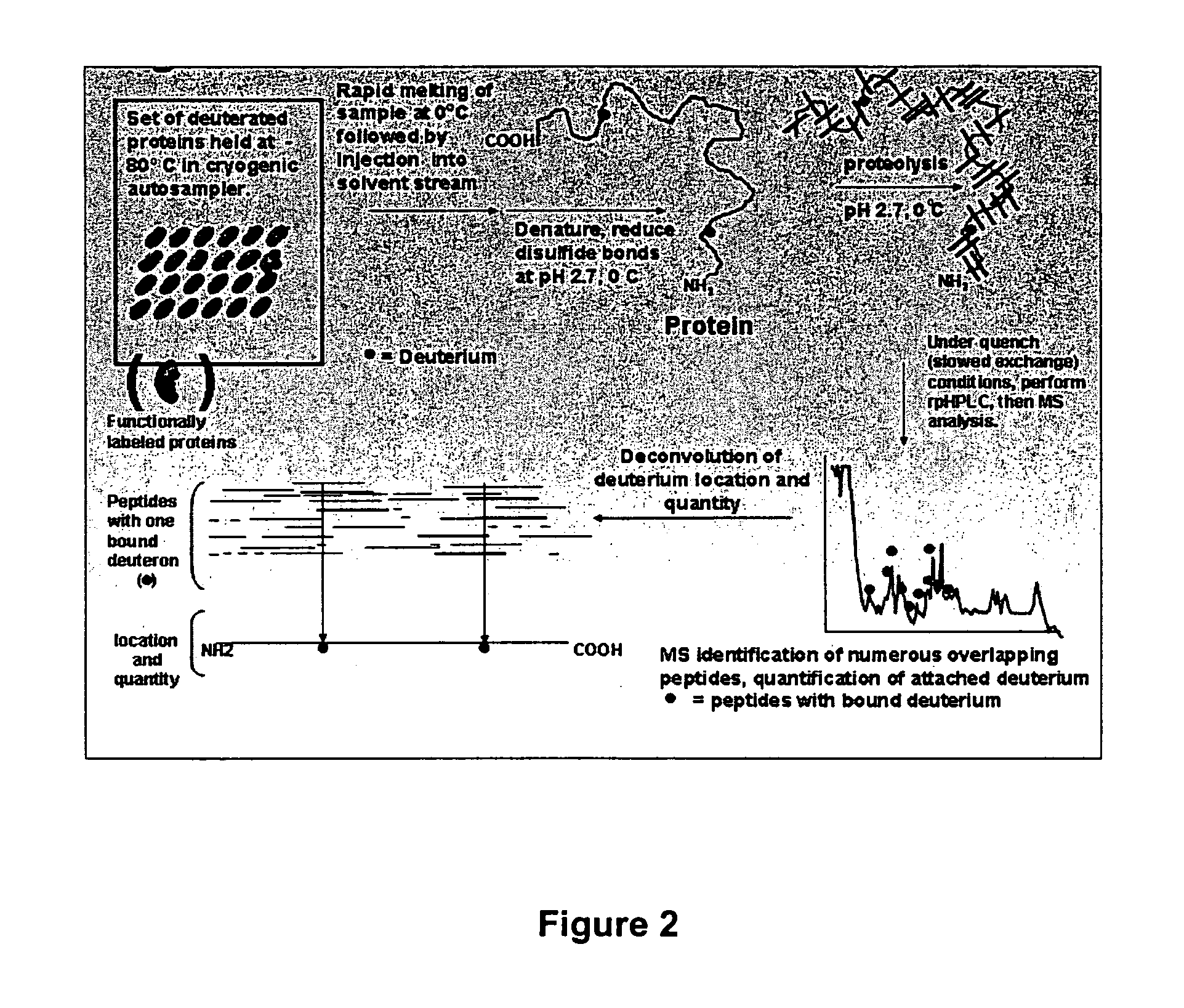
The present invention provides methods of structure prediction and / or determination of a protein of interest of unknown structure. Invention methods compare calculated rates of amide hydrogen exchange for a set of predicted possible structures for said protein with experimental hydrogen exchange analysis of said protein to identify valid protein structures based on similarities between hydrogen exchange profiles. In preferred methods, hydrogen exchange analysis is performed by determining the quantity of isotope and / or rate of exchange of peptide amide hydrogen(s) with isotope on a labeled protein, by generating a population of sequence-overlapping endopeptidase fragments of a protein labeled with a hydrogen isotope other than 1H under conditions of slowed hydrogen exchange, and then deconvoluting fragmentation data acquired from said population of sequence-overlapping endopeptidase-generated fragments.
Owner:THE UNIV OF TEXAS MEDICAL BRANCH +1
Precursor soot synthesis of fullerenes and nanotubes without formation of carbonaceous soot
The present invention is a method for the synthesis of fullerenes and / or nanotubes from precursor soot without the formation of carbonaceous soot. The method comprises the pyrolysis of a hydrocarbon fuel source by heating the fuel source at a sufficient temperature to transform the fuel source to a condensed hydrocarbon. The condensed hydrocarbon is a reaction medium comprising precursor soot wherein hydrogen exchange occurs within the reaction medium to form reactive radicals which cause continuous rearrangement of the carbon skeletal structure of the condensed hydrocarbon. Then, inducing dehydrogenation of the precursor soot to form fullerenes and / or nanotubes free from the formation of carbonaceous soot by continued heating at the sufficient temperature and by regulating the carbon to hydrogen ratio within the reaction medium. The dehydrogenation process produces hydrogen gas as a by-product. The method of the present invention in another embodiment is also a continuous synthesis process having a continuous supply of the fuel source. The method of the present invention can also be a continuous cyclic synthesis process wherein the reaction medium is fed back into the system as a fuel source after extraction of the fullerenes and / or nanotube products. The method of the present invention is also a method for producing precursor soot in bulk quantity, then forming fullerenes and / or nanotubes from the precursor bulk.
Owner:UT BATTELLE LLC
Method for preparing deuterated five-membered aromatic heterocyclic compound under catalysis of silver
InactiveCN110156766AStable deuterium rateImprove compatibilityIsotope introduction to heterocyclic compoundsChemical synthesisHydrogen–deuterium exchange
The invention belongs to the field of chemical synthesis, and concretely relates to a method for preparing a deuterated five-membered aromatic heterocyclic compound through a silver-catalyzed hydrogen-deuterium exchange reaction. Cheap deuterium oxide used as a deuteration reagent and dimethyl sulfoxide used as a solvent are subjected to the silver-catalyzed hydrogen-deuterium exchange reaction under a stirring condition at 25-120 DEG C to exchange hydrogen in the five-membered aromatic heterocycle with deuterium in order to conveniently synthesize the deuterated five-membered aromatic heterocyclic compound. The method has the advantages of mild conditions, avoiding of expensive transition metals and deuteration reagents in the reaction process, reduction of the production cost, and suitableness for large-scale production. The deuteration rate of the deuterated aromatic heterocyclic compound reaches 90% or more, the substrate application range is wide, and the compound can be applied to fields of organic photoelectric materials and medicinal pesticides.
Owner:NANJING UNIV OF TECH
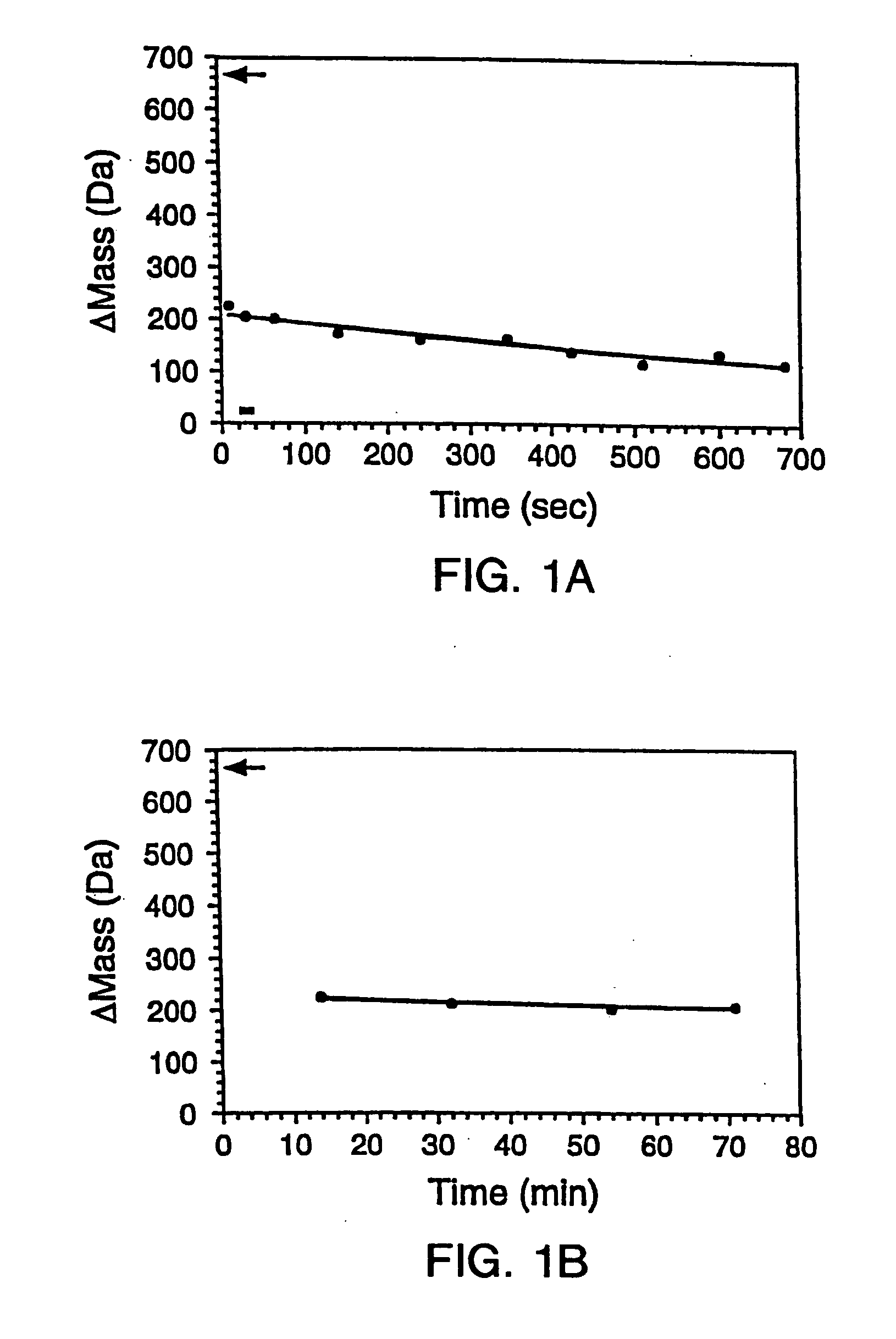
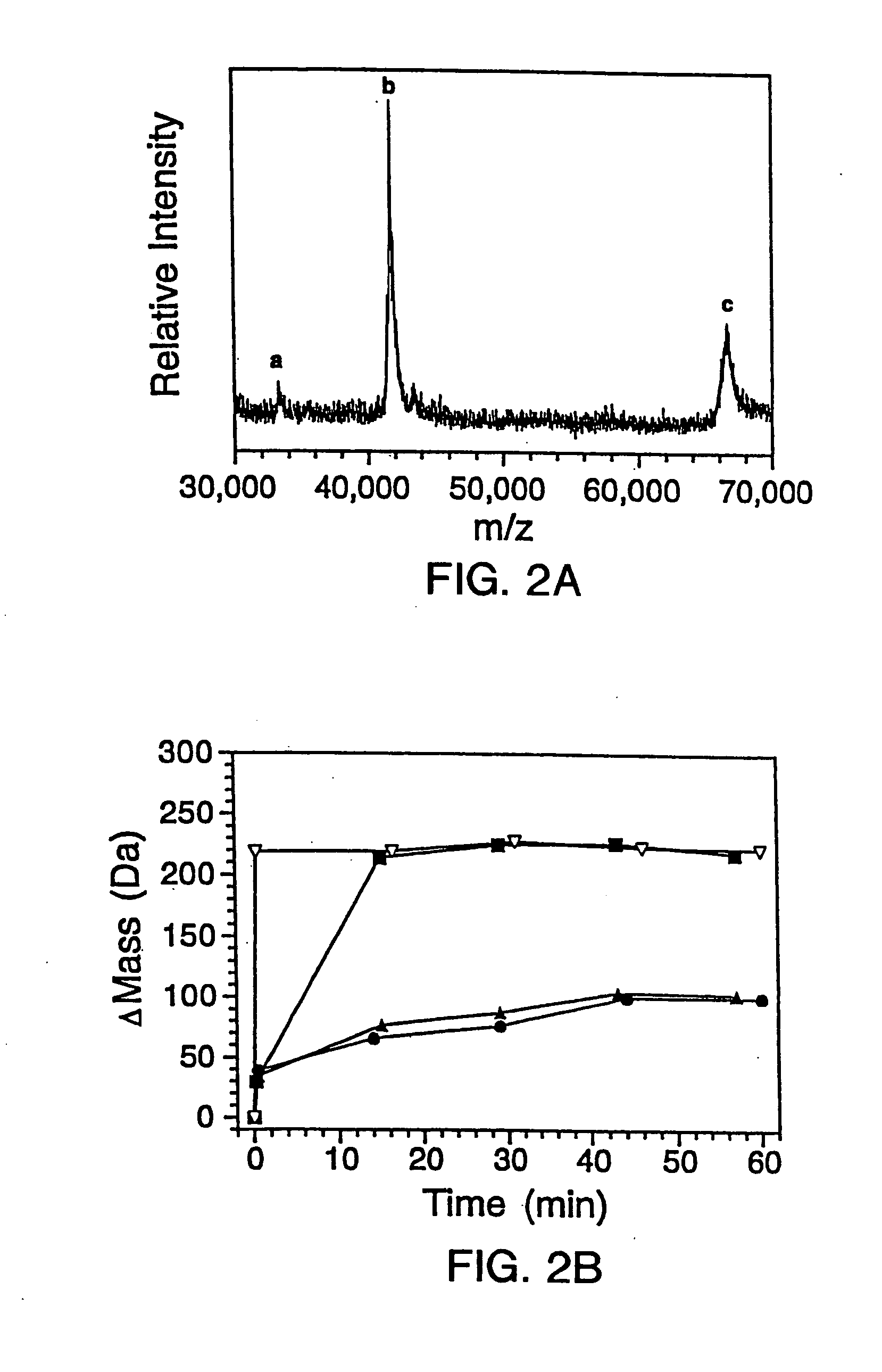
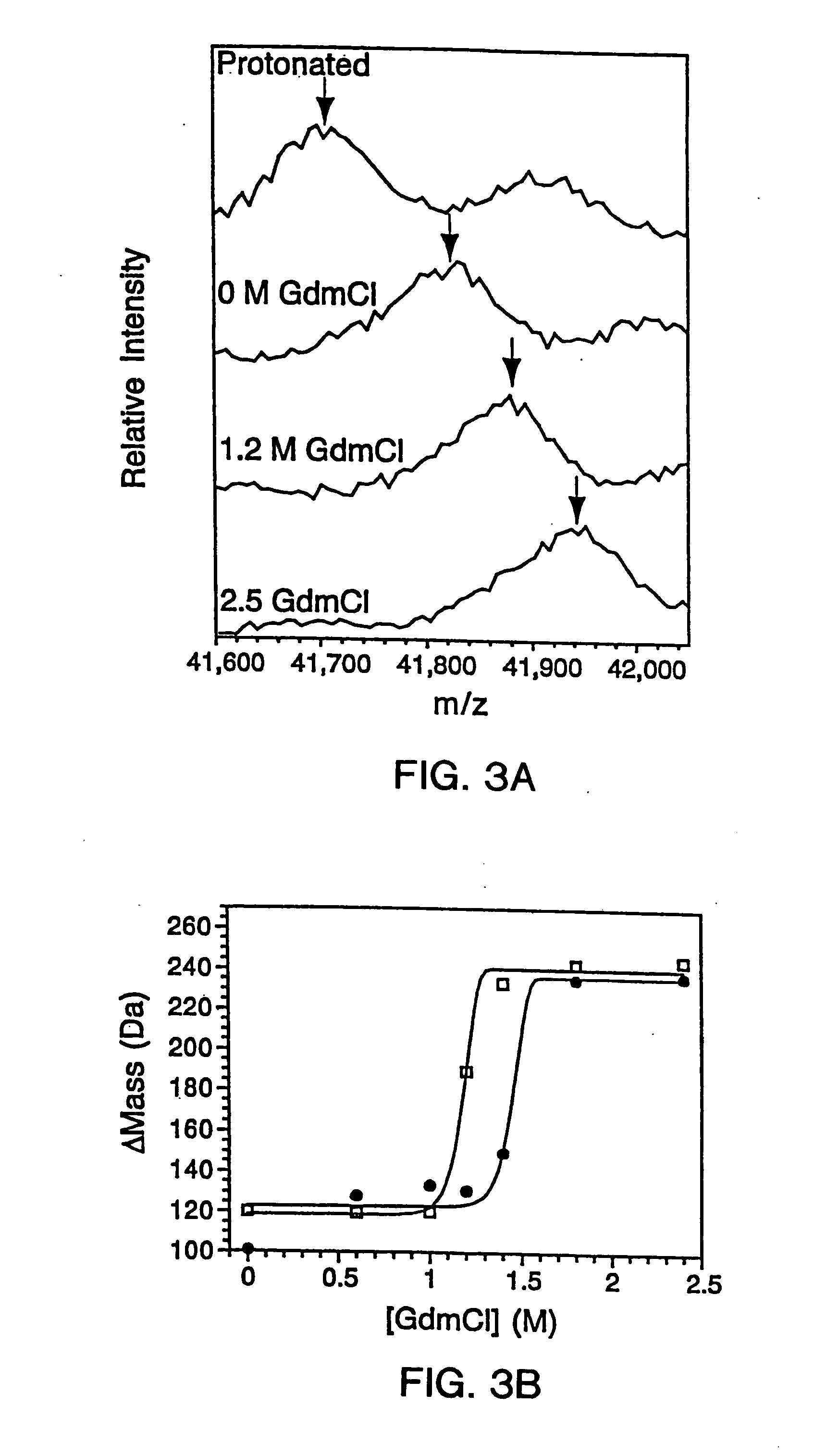
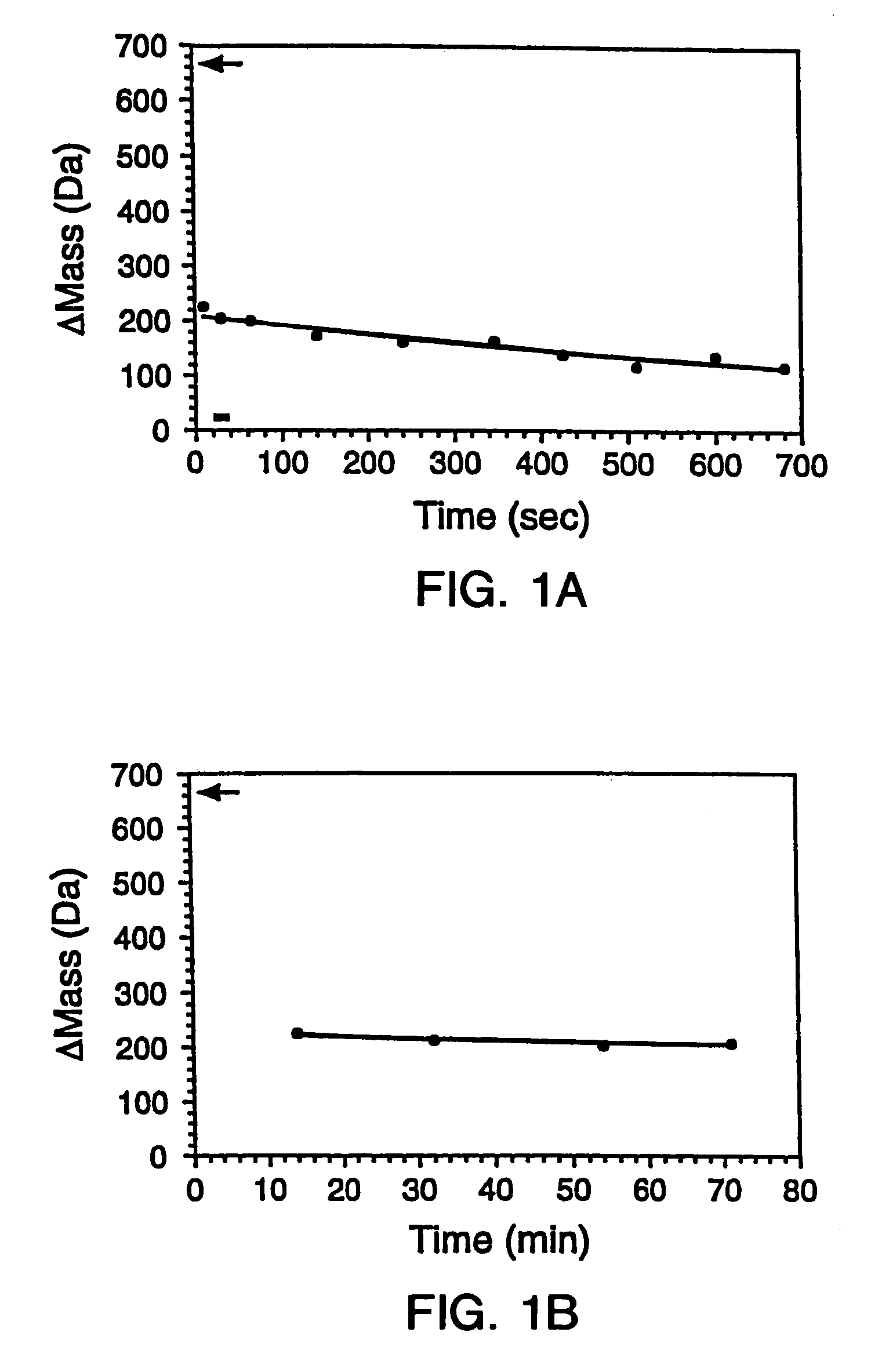
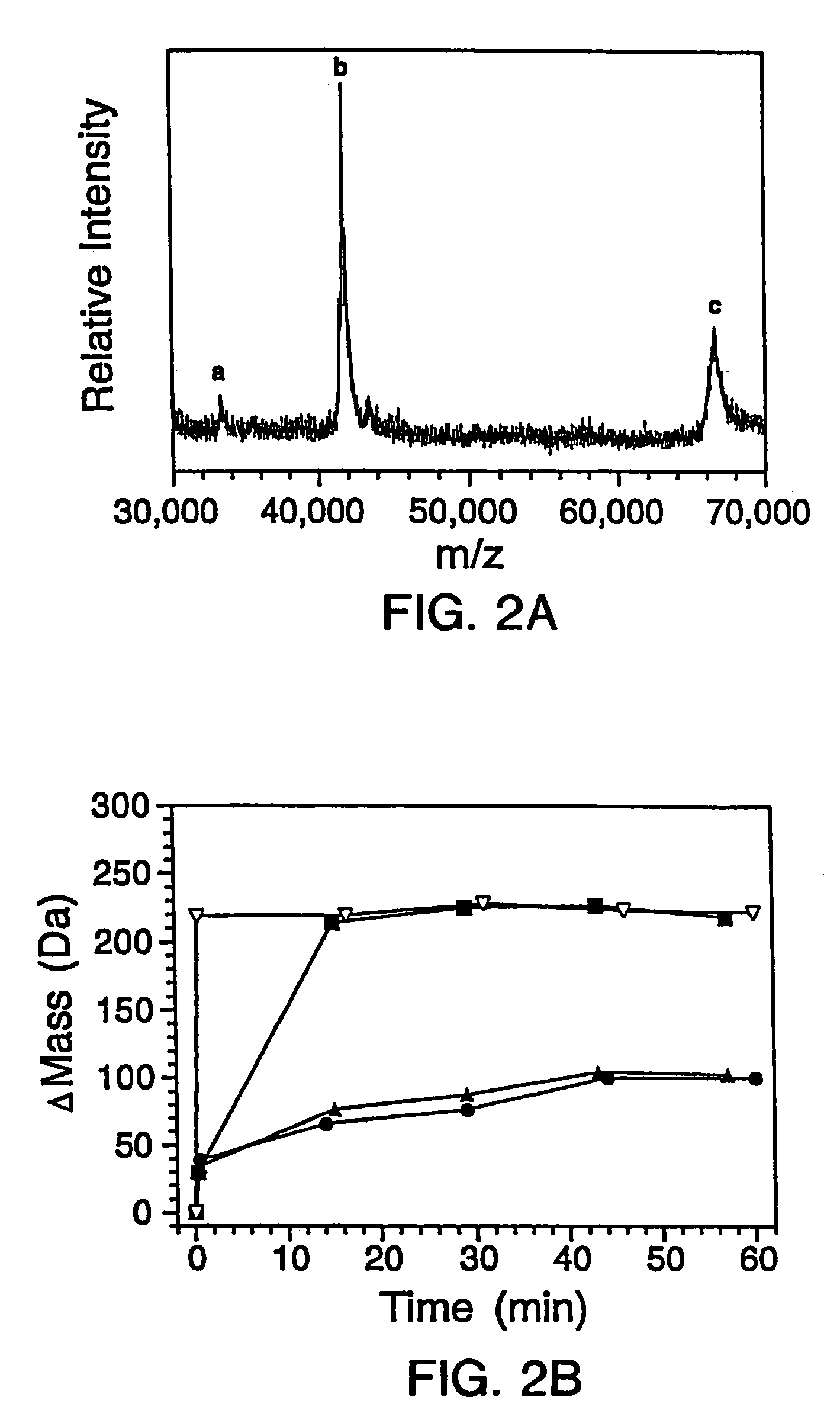
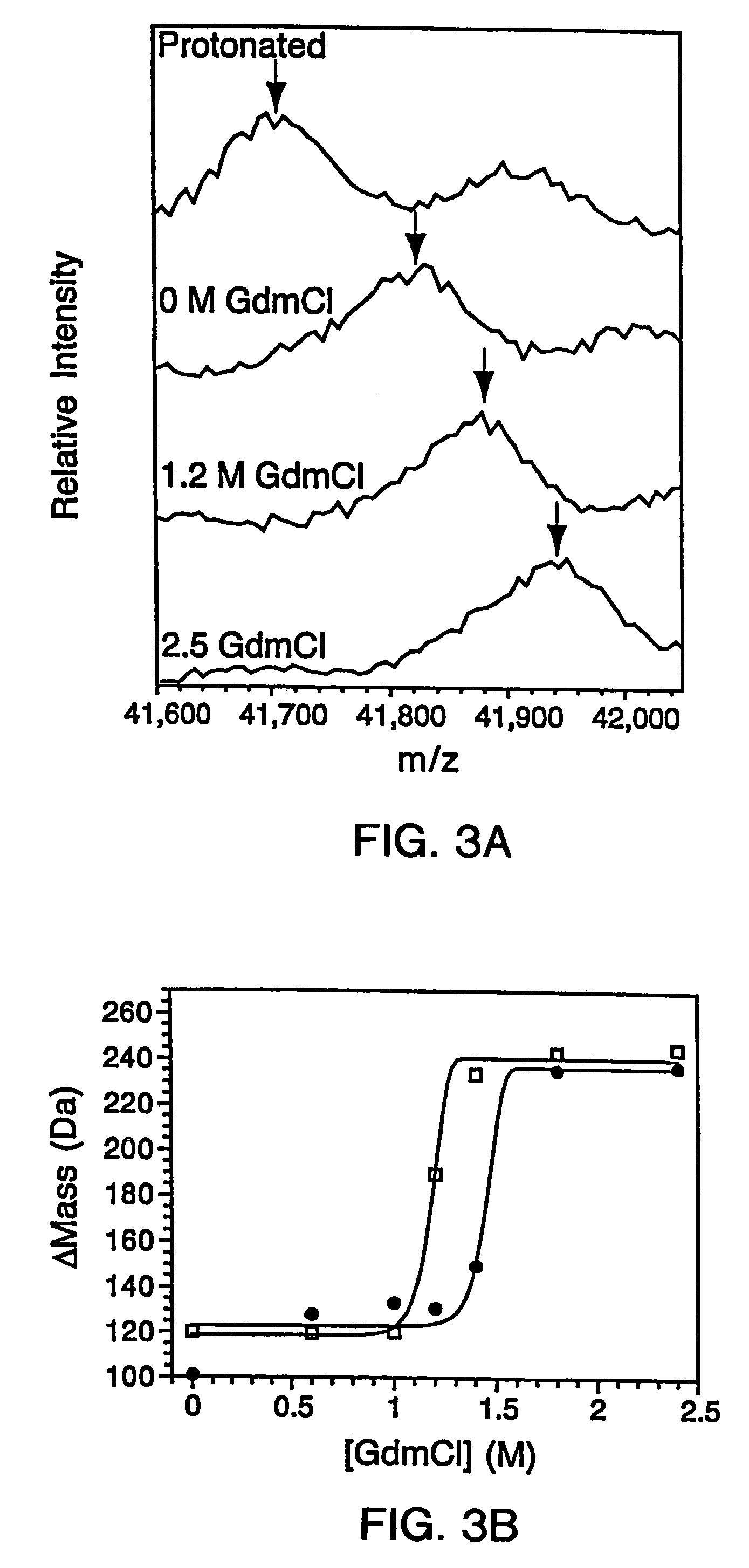
Quantitative, high-throughput screening method for protein stability
InactiveUS20070099305A1Improve completenessImprove stabilityDisease diagnosisBiological testingLaser desorption ionization mass spectrometryHigh-Throughput Screening Methods
In proteomic research, it is often necessary to screen a large number of polypeptides for the presence of stable structure. Described herein are methods (referred to as MALDI MS-HX and SUPREX) for measuring the stability of proteins in a rapid, high-throughput fashion. The method employs hydrogen exchange to estimate the stability of quantities of unpurified protein extracts, using matrix-assisted laser desorption / ionization (MALDI) mass spectrometry. A method of quantitatively determining the stability of a test protein under native conditions is disclosed. The method includes the steps (a) providing a test protein; (b) contacting the protein with an exchange buffer comprising a denaturant and deuterium, the exchange buffer having a denaturant concentration; (c) contacting the test protein with a mass spectrometry matrix medium; (d) determining a change in mass of the test protein by mass spectrometry; (e) varying the denaturant concentration of the exchange buffer; (f) repeating steps (a)-(e) a desired number of times; and (g) quantitatively determining protein stability based on the change in mass of the test protein as a function of denaturant concentration, whereby the stability of a test protein under native conditions is quantitatively determined.
Owner:DUKE UNIV
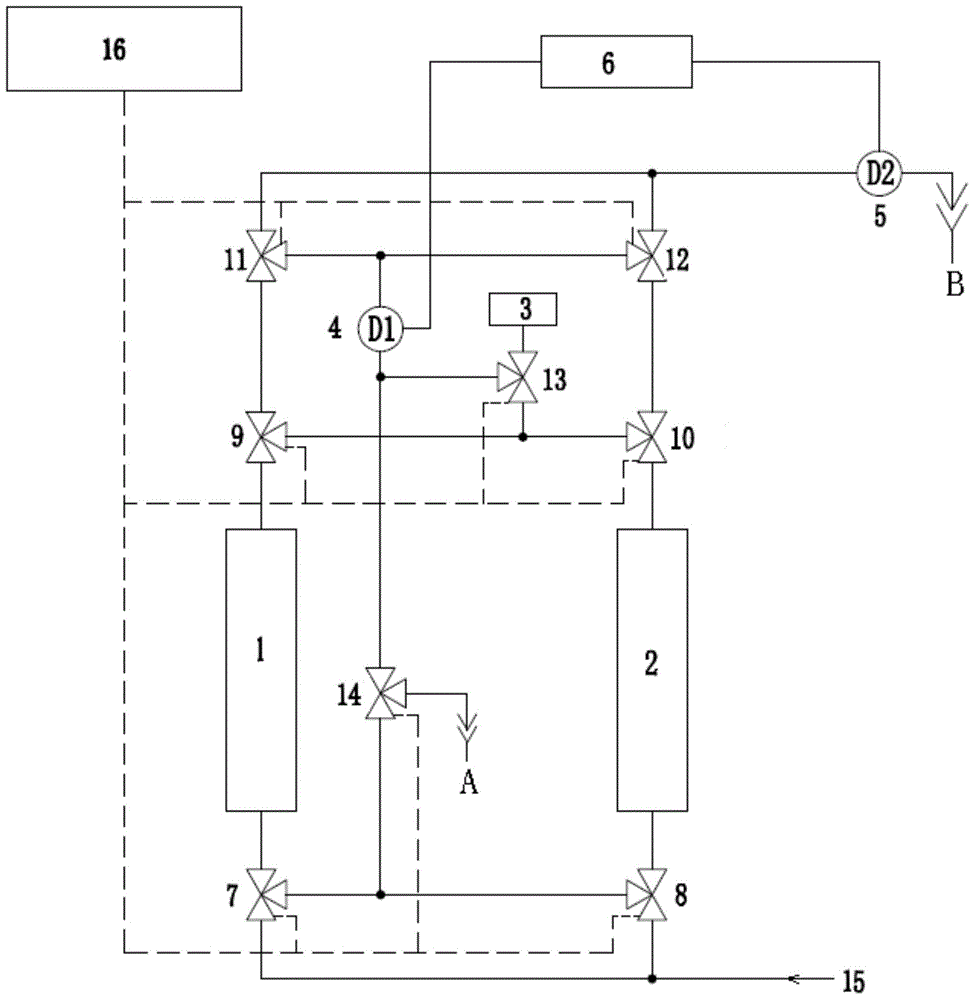
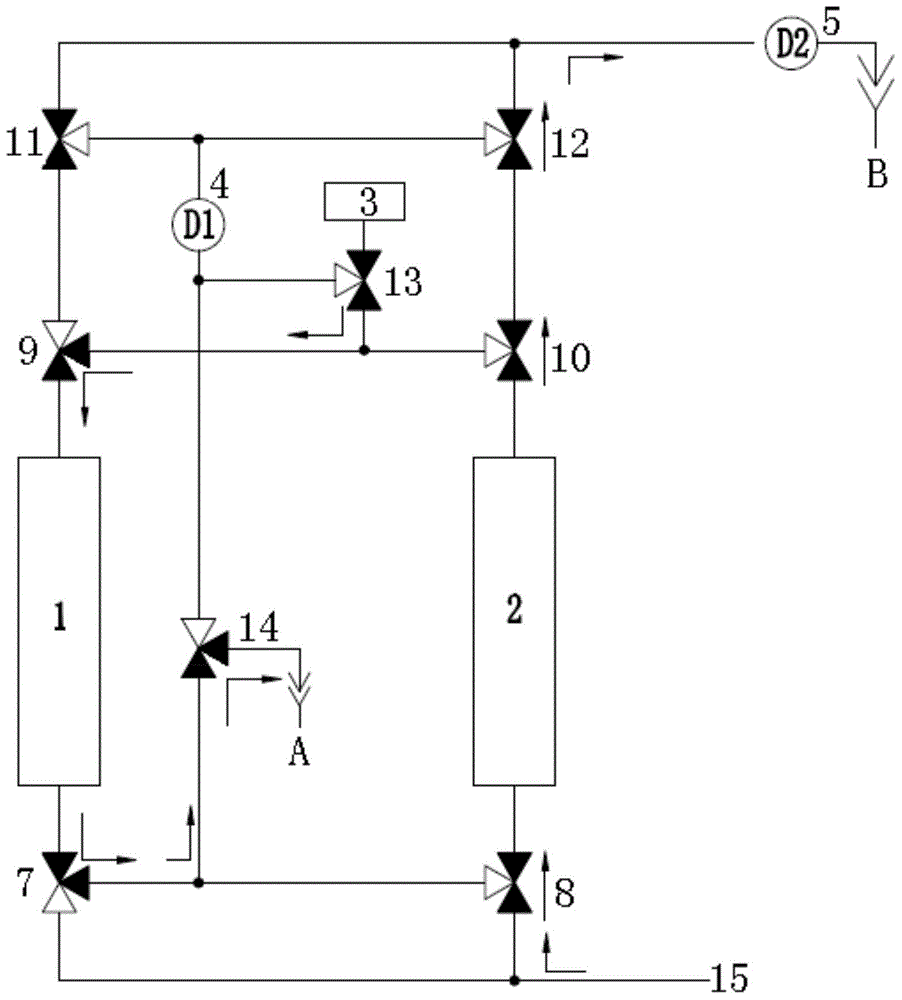
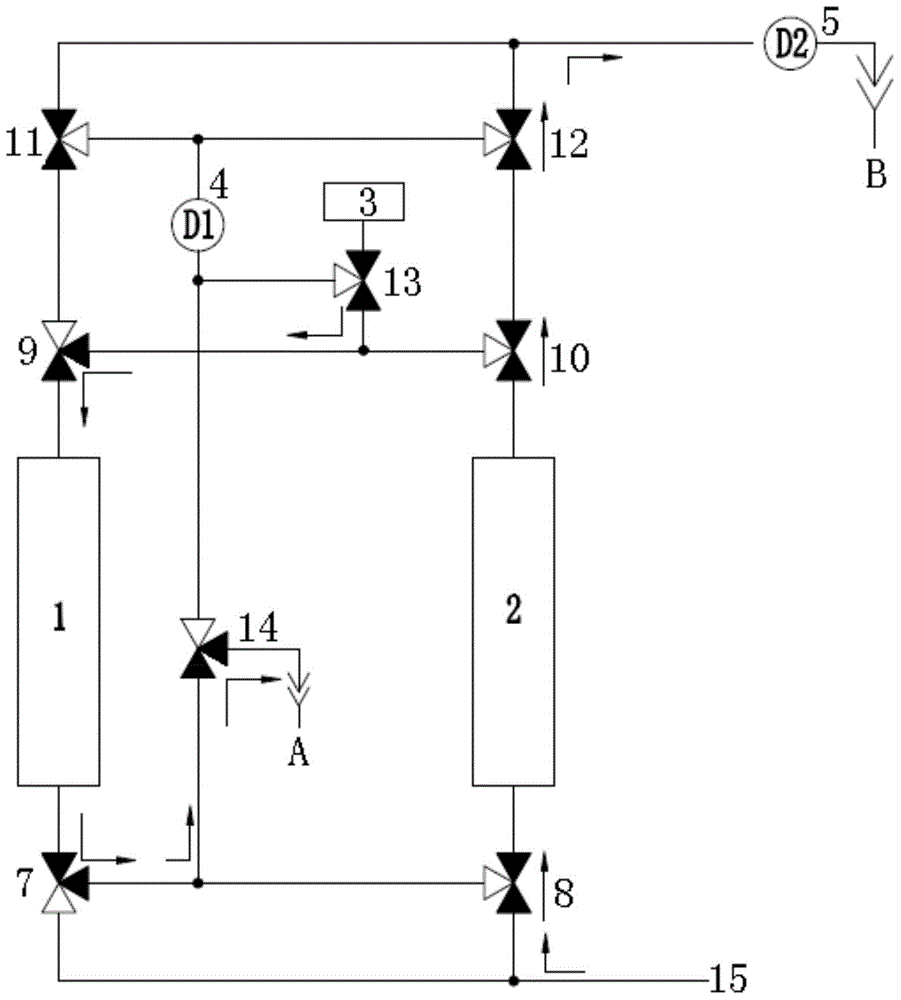
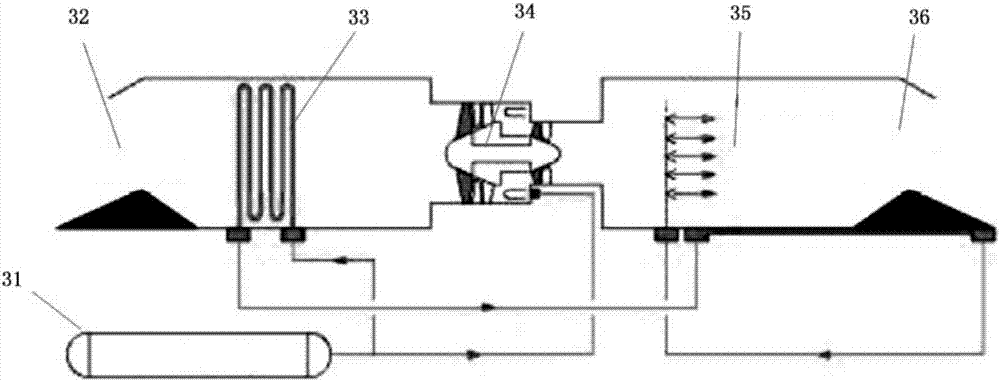
Continuous online hydrogen conductivity measurement device
InactiveCN104614590AUninterrupted continuous and accurate measurementSolve problems that cannot be measured normallyResistance/reactance/impedenceMeasurement deviceHydrogen exchange
The invention provides a continuous online hydrogen conductivity measurement device. The continuous online hydrogen conductivity measurement device comprises a hydrogen exchange column 1# and a hydrogen exchange column 2# which are arranged in parallel, hydrogen-type cation exchange resin is arranged in the hydrogen exchange column 1# and the hydrogen exchange column 2#, the water inlet ends of the hydrogen exchange column 1# and the hydrogen exchange column 2# are respectively connected with water inlet pipelines through respective connecting pipes, and the water outlet ends of the hydrogen exchange column 1# and the hydrogen exchange column 2# are respectively connected with a dual-channel online hydrogen conductivity meter through respectively connecting pipe. An electrical conductivity D1 and an electrical conductivity D2 are respectively arranged on the water outlet pipelines of the hydrogen exchange column 1# and the hydrogen exchange column 2#. The measurement device further comprises a liquid regeneration system, wherein the liquid regeneration system is connected with the hydrogen exchange column 1# and the hydrogen exchange column 2# respectively. The continuous online hydrogen conductivity measurement device can achieve continuous online hydrogen conductivity measurement of a water sample, automatically judge resin invalidation, automatically performs online regeneration on invalid resin, performs automatic flushing and is switched to be in a standby state after completing regeneration, and the problem that the hydrogen conductivity of the water sample cannot be measured during resin replacement is solved.
Owner:HKY TECH
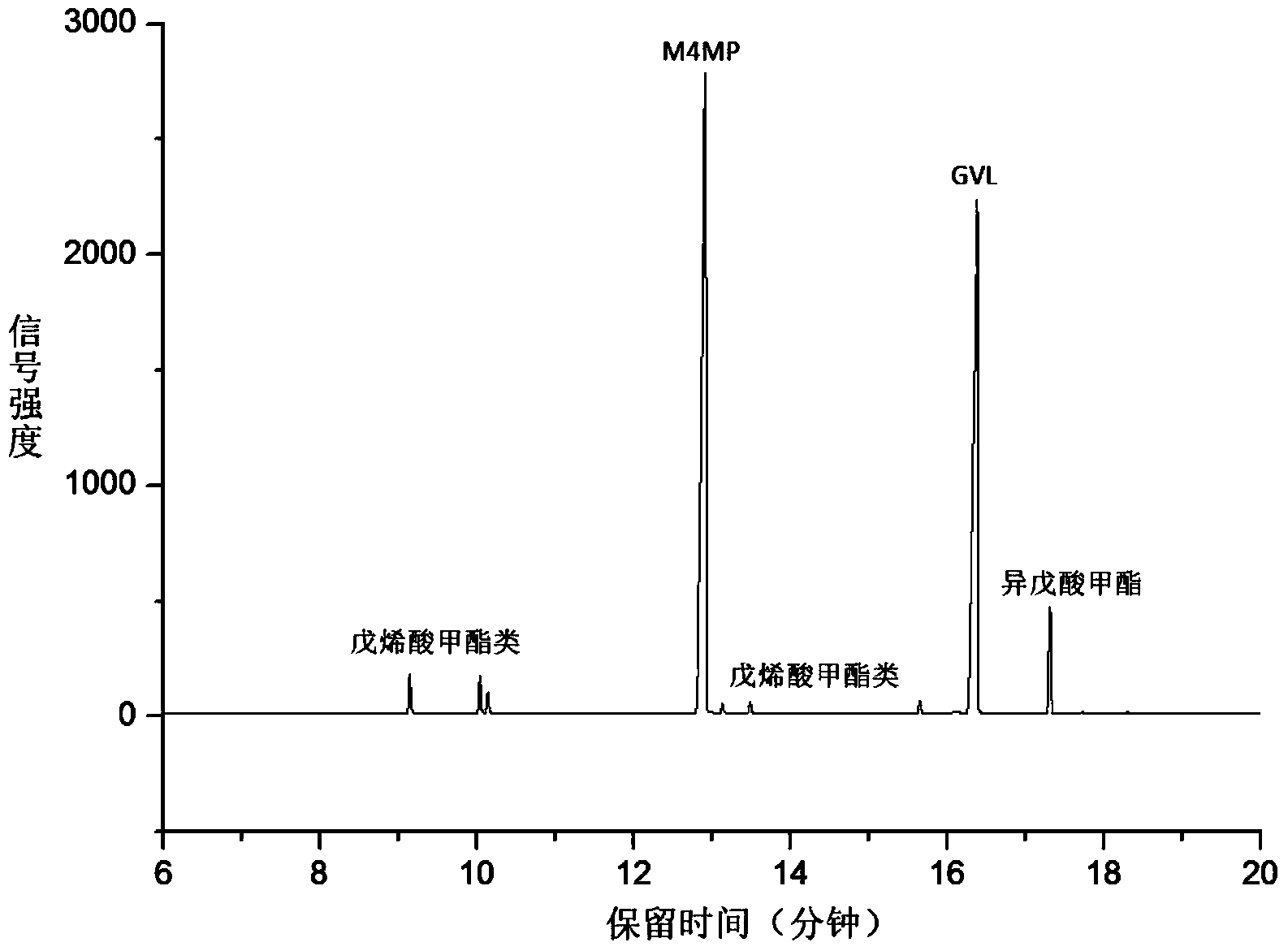
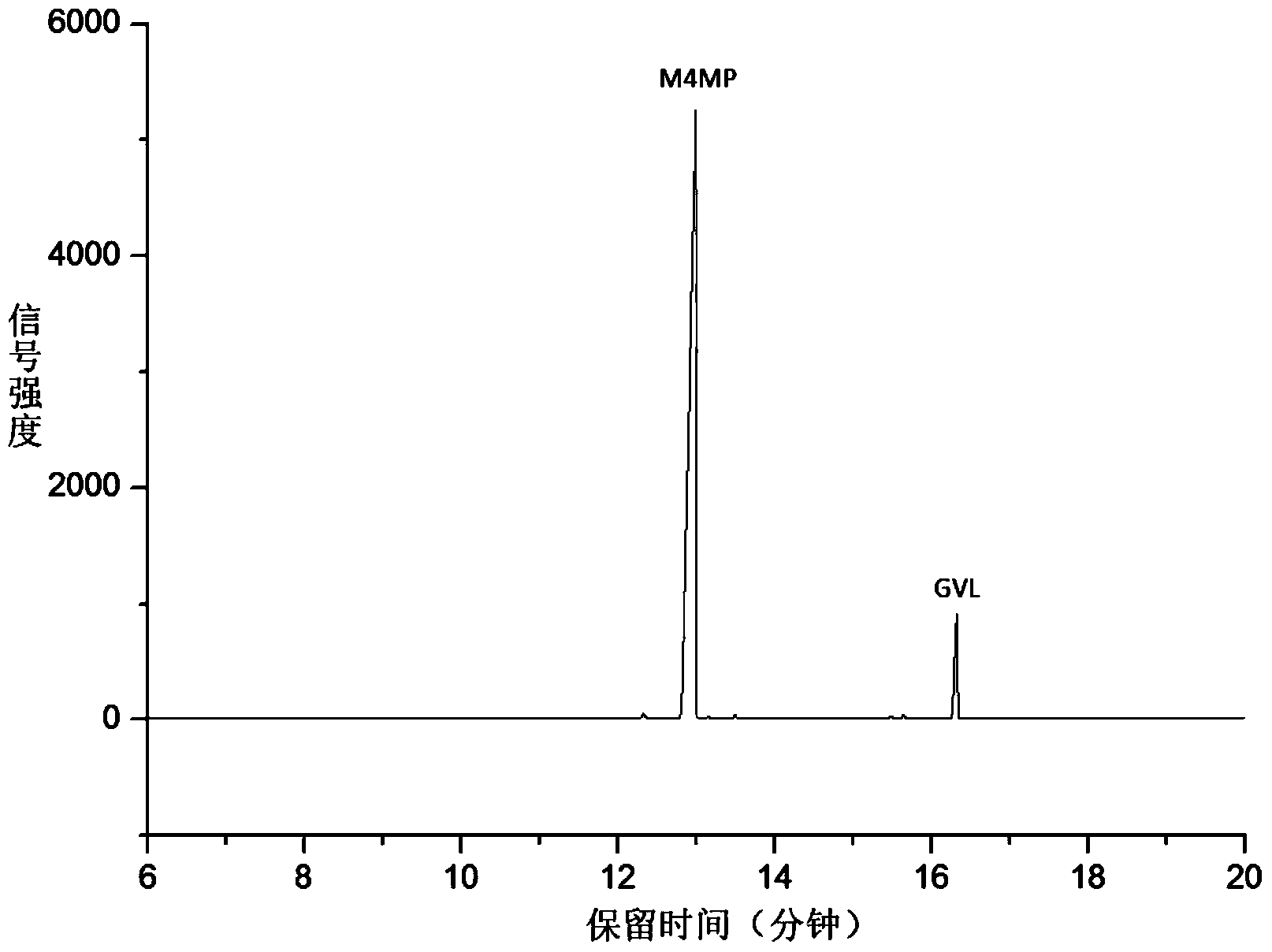
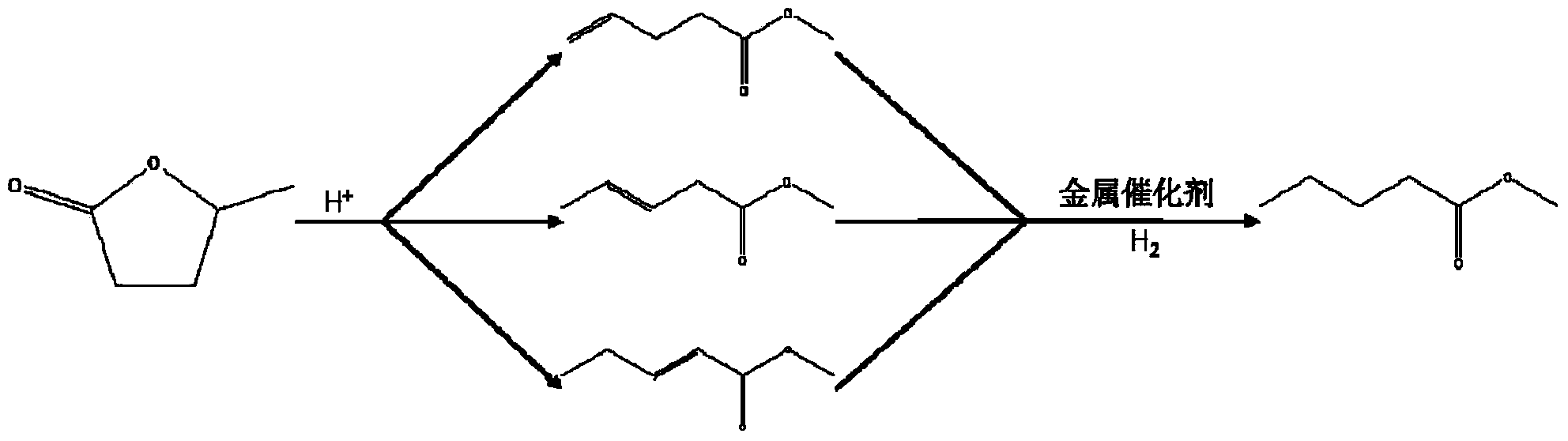
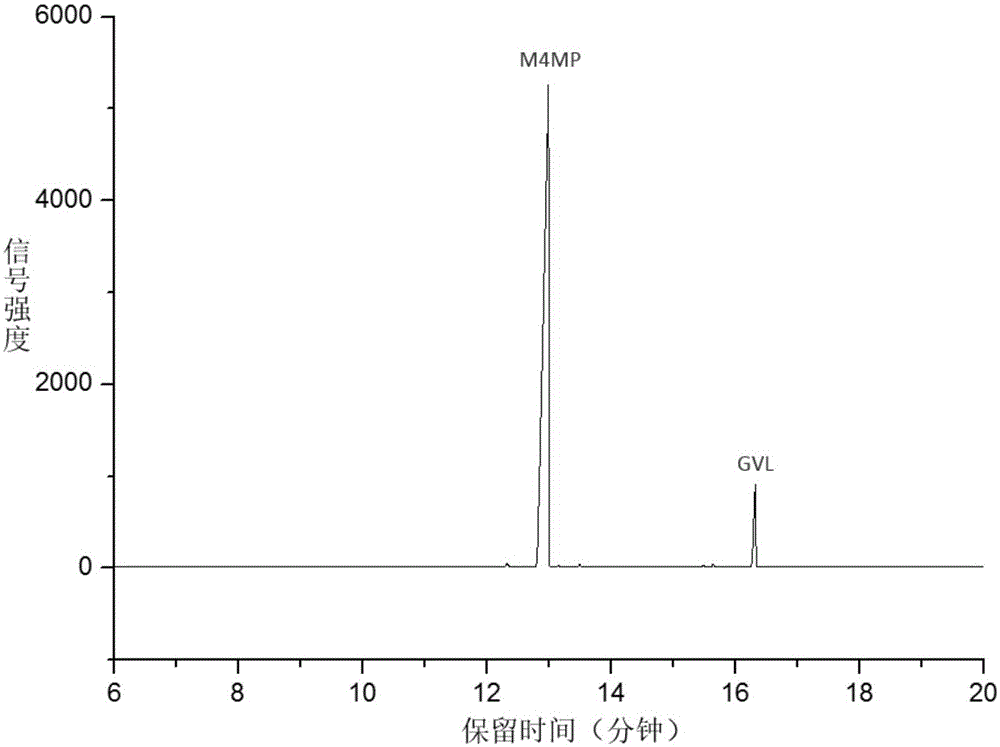
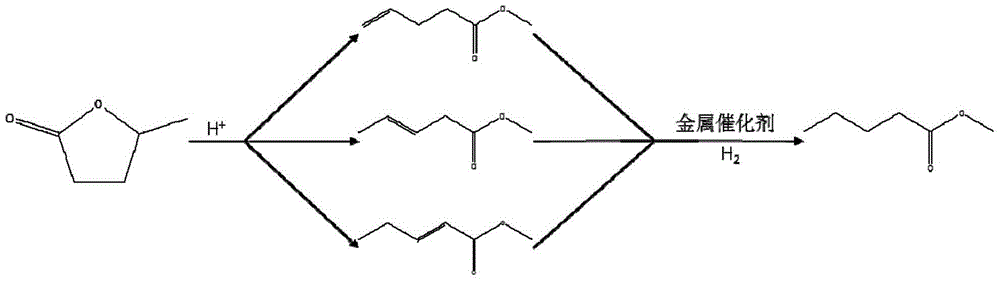
Method for preparing methyl 4-methoxy valerate from gamma-valerolactone
ActiveCN104341294AImprove performanceGreat application potentialMolecular sieve catalystsPreparation by ester-hydroxy reactionFiltrationDistillation
The invention discloses a method for preparing methyl 4-methoxy valerate from gamma-valerolactone and relates to methyl 4-methoxy valerate. The invention provides a low-cost and environment-friendly method for preparing methyl 4-methoxy valerate from gamma-valerolactone. The method comprises the following steps: (1) adding a hydrogen-exchange ultrastable Y-type molecular sieve and CaCO3 as catalysts into a mixed solution of gamma-valerolactone and methanol and reacting under an inert gas atmosphere to obtain a solid-liquid mixture; and (2) carrying out reduced pressure suction filtration on the solid-liquid mixture obtained in the step (1) to obtain a mixed solution and carrying out reduced pressure distillation on the obtained mixed solution to obtain methyl 4-methoxy valerate. Methyl 4-methoxy valerate is synthesized in a high yield from gamma-valerolactone and methanol by synergetic catalysis of the hydrogen-exchange ultrastable Y-type molecular sieve and CaCO3 for the first time and the method provides an entirely new way to biomass synthesis of high value-added chemicals.
Owner:XIAMEN UNIV
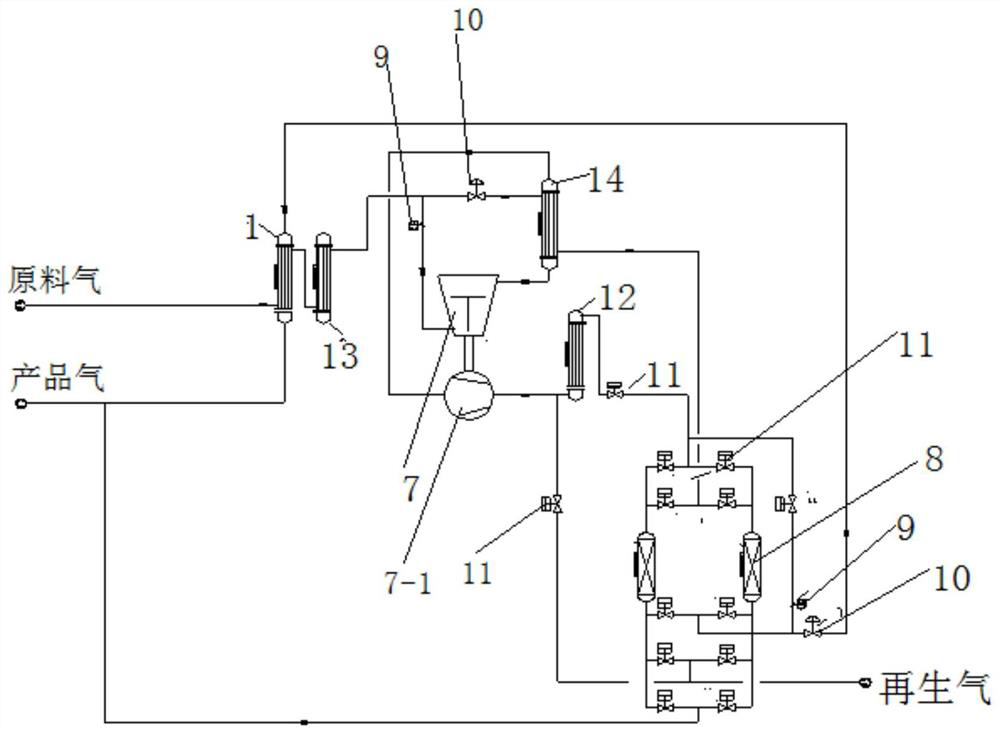
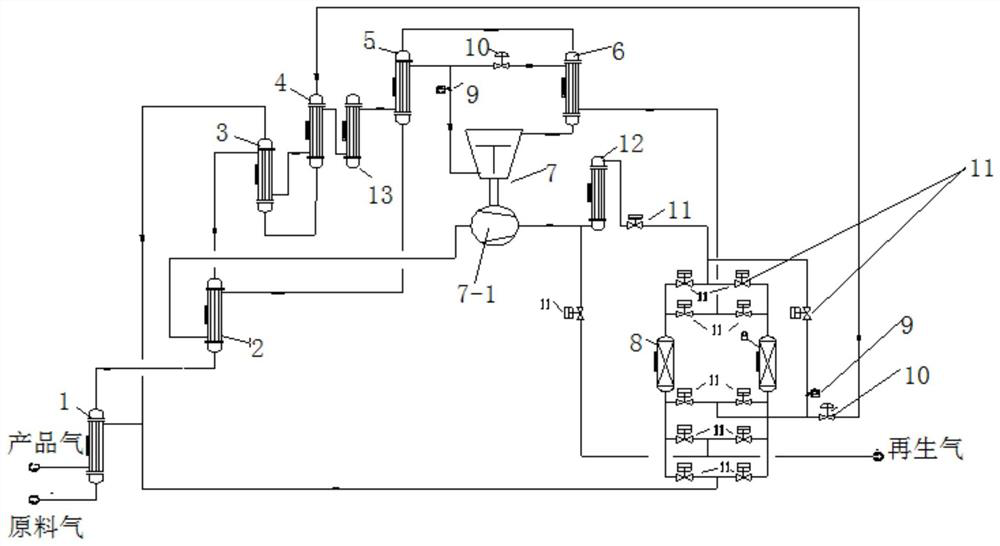
Method and system device for preparing hydrogen for purifying fuel cell
PendingCN112201824AGuaranteed uptimeHigh purification precisionHydrogen separationHydrogen/synthetic gas productionFuel cellsHydrogen exchange
The invention relates to the field of hydrogen purification, in particular to a method and a system device for preparing hydrogen for purifying a fuel cell. Raw material hydrogen exchanges heat with product gas and regeneration gas after passing through a heat exchange mechanism I, namely an N-stage heat exchanger, and after the temperature is reduced, a part of hydrogen is separated out and enters a refrigerating mechanism to obtain supercooled hydrogen; the raw material hydrogen and the supercooled hydrogen are cooled by a heat exchange mechanism II and then enter an adsorption mechanism, and impurities in the raw material hydrogen are purified by a low adsorption mechanism until the requirements of hydrogen for the fuel cell are met, so that hydrogen product gas for the fuel cell is obtained; cold hydrogen is heated by a heater, the heated hydrogen is used as a medium for regeneration reconstitution and thermal regeneration of the low-temperature adsorption mechanism, and the purified product gas returns to the heat exchange mechanism I to exchange heat with the raw material hydrogen, is heated and then is output to storage equipment or other treatment equipment. The impurity content S in the obtained product gas is less than 0.004 ppm, the CO content is less than 0.02 ppm, and the long-period stable operation of the fuel cell is ensured.
Owner:SOUTHWEST RES & DESIGN INST OF CHEM IND
Method for fast exchanging hydrogen after urgency purging of bell-type furnace
ActiveCN103451408AReduce oven timeReduce oxidationFurnace typesHeat treatment furnacesSurface oxidationHydrogen exchange
The invention provides a method for fast exchanging hydrogen after the urgency purging of a bell-type furnace. The method comprises the following steps: manufacturing a simulating heating cover signal plug; programming a fast hydrogen exchange program of an annealing control computer; setting nitrogen purging time and heating cover ignition time to 0, and setting hydrogen exchange time to 12 minutes; controlling furnace atmosphere O2 to be less than 1; when the hydrogen is fast exchanged, firstly disassembling a cooling cover signal plug, connecting the simulating heating cover signal plug, and then starting a heating program to automatically carry out hydrogen exchange according to the set time; and after the hydrogen exchange is finished, disassembling the simulating heating cover signal plug, connecting the cooling cover signal plug, and continuously cooling. According to the method provided by the invention, the integral hydrogen exchange process only needs 30 minutes; the surface oxidation of a steel plate can be effectively reduced, the surface quality of the steel plate can be enhanced, and the second annealing repair of an oxidized steel plate can be avoided; the energy resources are greatly saved, and the production cost is reduced.
Owner:ANGANG STEEL CO LTD
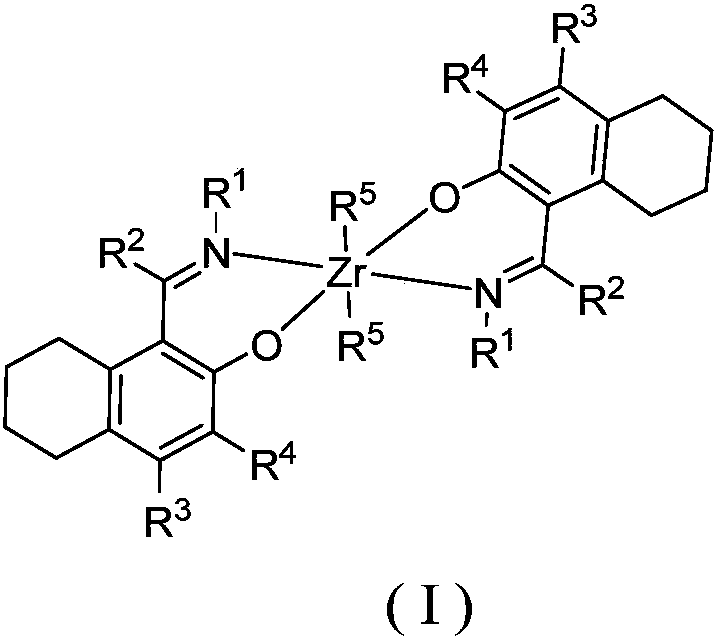
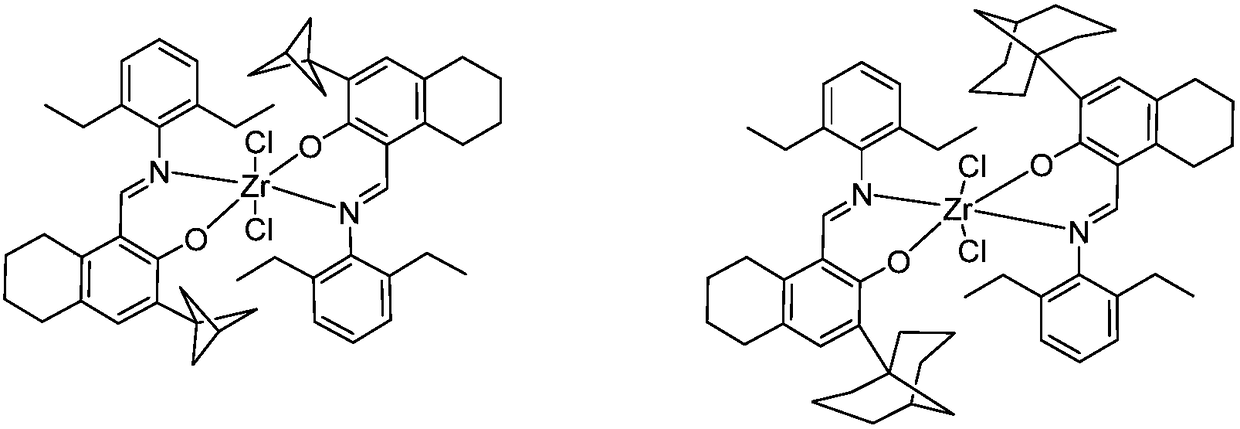
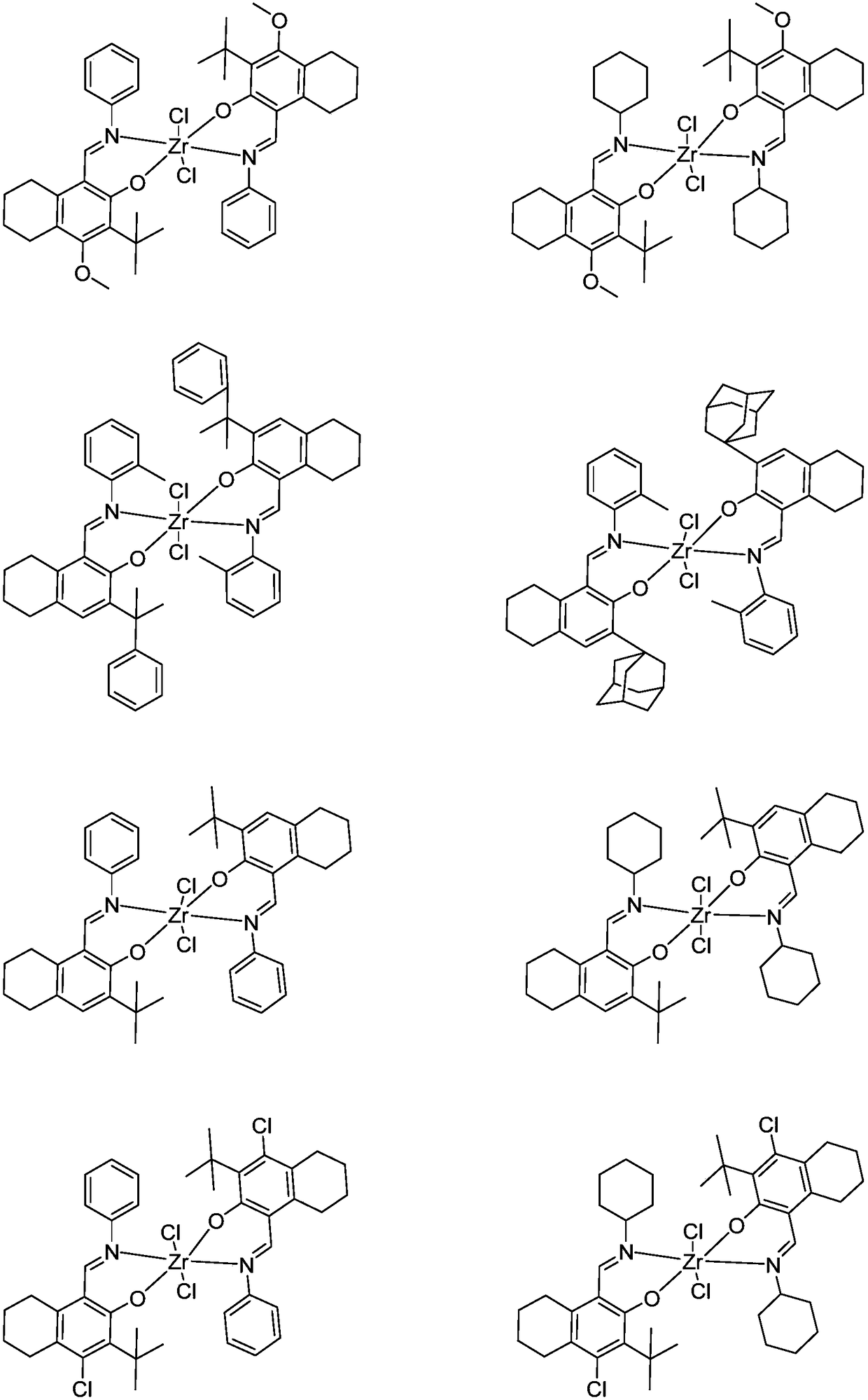
Zirconium tetralylmethoxyl imine complex and preparation method and application thereof
InactiveCN108383864AEasy to manufactureStable in natureSilicon organic compoundsBulk chemical production1-HexenePolypropylene
The invention relates to a zirconium tetralylmethoxyl imine complex, a preparation method thereof, and application thereof in catalysis olefin homopolymerisation and copolymerization. In the preparation method, tetrahydronaphthol imine neutral ligand is subjected to hydrogen exchange treatment with lithium alkylide to obtain ligand lithium salt, the ligand lithium salt and zirconium halide react to obtain a product, the product is then filtered, concentrated and recrystallized to obtain the target complex finally. In the presence of a promoter, the zirconium complex can be used for catalyzingreactions such as ethylene and propylene homopolymerisation and copolymerization between ethylene and propylene, ethylene and 1-hexene, ethylene and 1-octylene, and ethylene and norbornene. Accordingto the zirconium Tetralylmethoxyl imine complex, the preparation method and application thereof, the complex has the advantages that raw materials are easy to obtain, synthesis routes of the complex are simple, yield of products is high, property of the complex is stable, the complex has high catalytic activity and can be used for preparing polyethylene, polypropylene, and copolymers of ethylene and propylene, ethylene and 1-hexene, ethylene and 1-octylene, and ethylene and norbornene which have high molecular weight and narrow range of the molecular weight, and can meet requirements of industrial sectors.
Owner:SHANGHAI RES INST OF CHEM IND
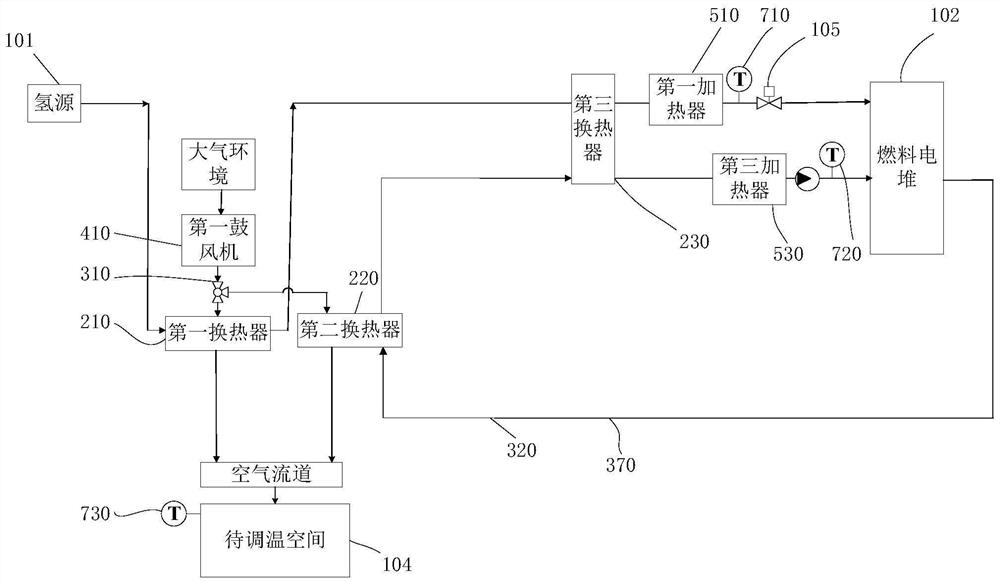
Fuel cell and thermal management control system thereof
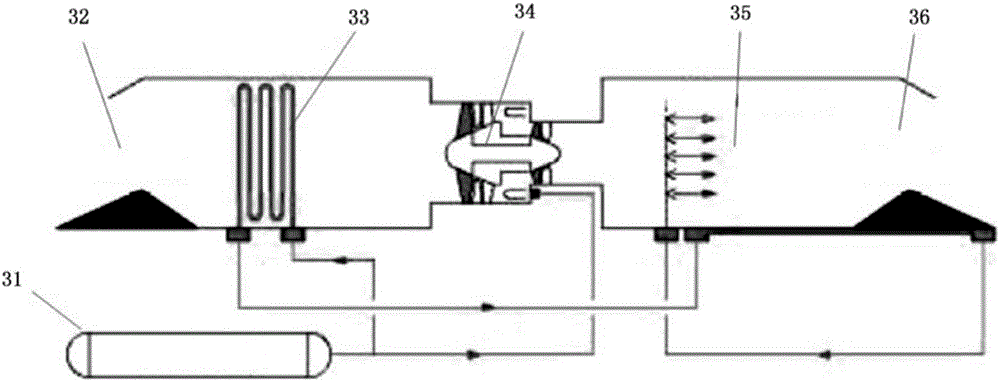
The invention relates to a fuel cell and a thermal management control system thereof.The thermal management control system at least comprises a hydrogen supply pipeline and a cooling liquid loop, at least one heat exchanger is arranged between a hydrogen storage tank of the hydrogen supply pipeline and an anode of a galvanic pile, and one end of the heat exchanger is connected with a cooling outlet of the galvanic pile; the other end of the heat exchanger is connected with a cooling inlet of a galvanic pile through a pump, under the condition that hydrogen exchanges heat with a cooling medium through the heat exchanger, the hydrogen absorbs heat energy of the cooling medium to be gasified, and the hydrogen with the increased pressure drives a turbine pressure reduction mechanism to perform pressure reduction action; and / or the heat exchanger further enables the refrigerant to communicate with the air conditioning system loop in a mode of passing through the heat exchanger, so that the refrigerant in the air conditioning system loop exchanges heat energy with the hydrogen. The method has the advantages that firstly, the cold energy of the liquid hydrogen is effectively utilized; and secondly, by arranging the heat exchanger, the power of the radiator is reduced or even the radiator is omitted, and energy consumption is saved.
Owner:SHANGHAI EVERPOWER TECHNOLOGIES LTD
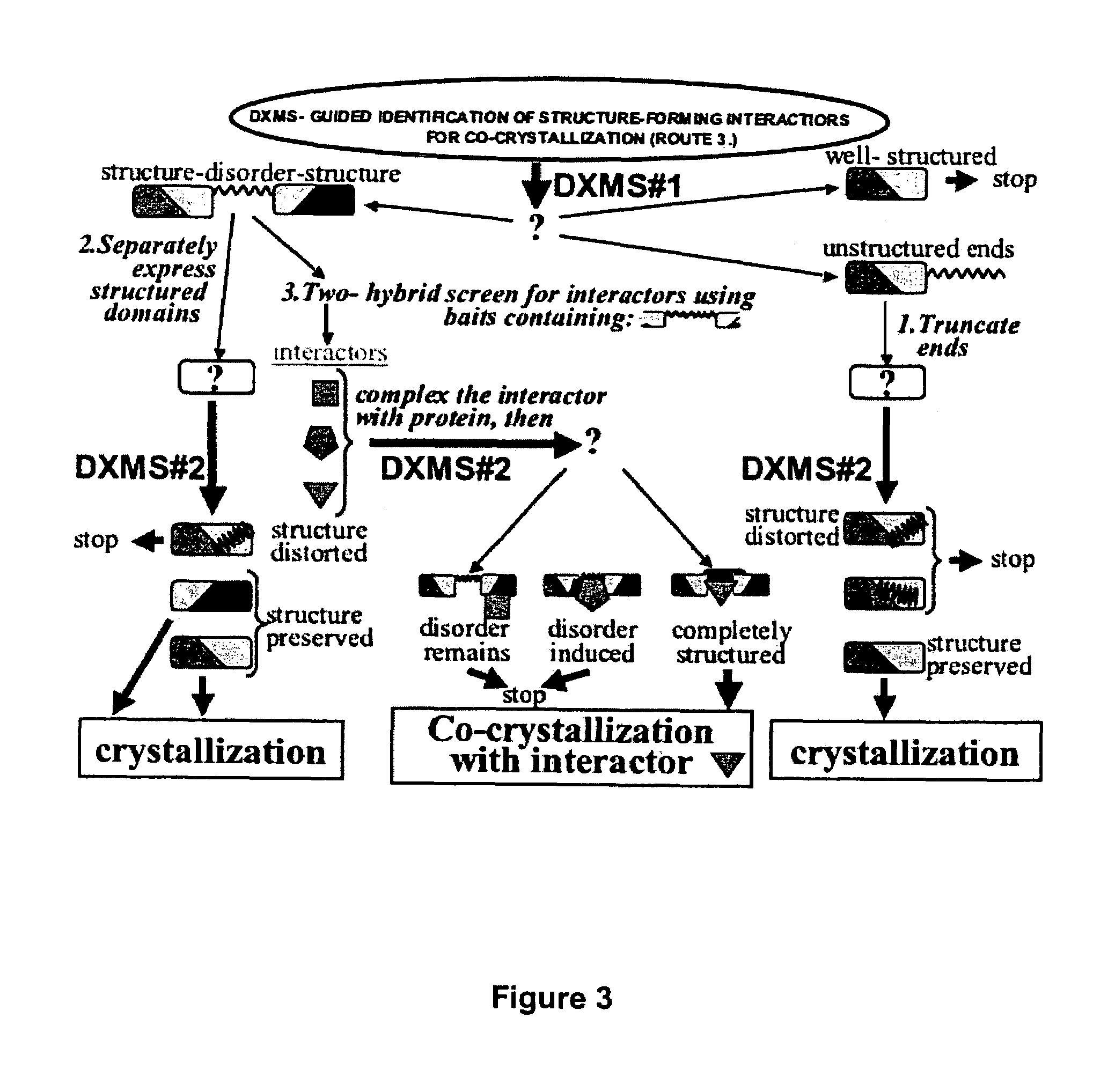
Methods for crystallographic structure determination employing hydrogen exchange analysis
InactiveUS7280923B2High resolutionSimple methodMaterial analysis using wave/particle radiationBiological testingHydrogen exchangeCrystal structure
The present invention provides methods for crystallographic structure determination employing hydrogen exchange analysis. Hydrogen exchange analysis is used to identify unstructured regions of a protein, which are then deleted to obtain one or more modified proteins for crystallization and structure determination. Optionally, an initial hydrogen exchange stability map of a protein is compared to that of at least one modified form of the protein to identify unstructured regions, while retaining characteristic structure of the native protein. Hydrogen exchange analysis is performed by determining the quantity of isotope and / or rate of exchange of peptide amide hydrogen(s) with isotope on a labeled protein. Proteins with fewer unstructured regions, and thus improved hydrogen exchange structural maps, are optimal for high quality crystallization and structure determination.
Owner:RGT UNIV OF CALIFORNIA
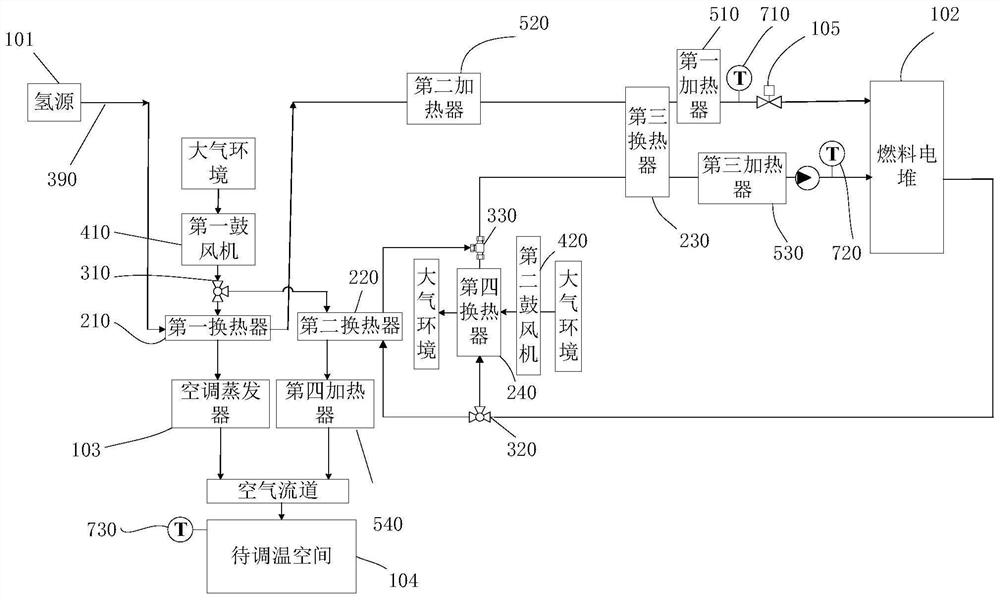
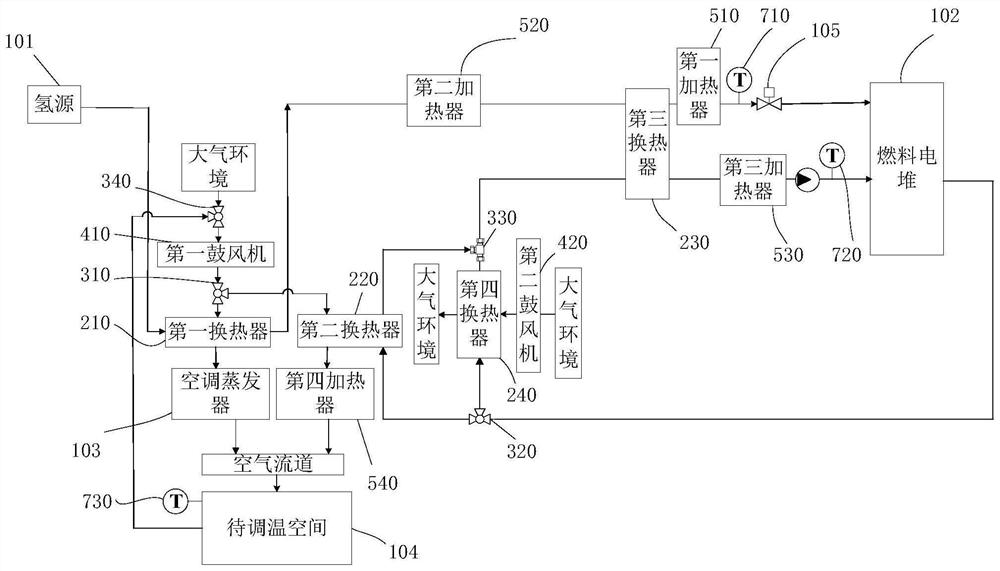
Fuel cell system and fuel cell vehicle
InactiveCN112599814ARealize comprehensive utilizationIncrease profitFuel cell heat exchangeReactant parameters controlFuel cellsHydrogen exchange
The invention relates to a fuel cell system and a fuel cell vehicle. The fuel cell system comprises a first heat exchanger, a first air blower, a first valve, a second heat exchanger and a third heatexchanger, wherein low-temperature hydrogen exchanges heat with air through the first heat exchanger, low-temperature air enters a space to be subjected to temperature adjustment to achieve the cooling of the space to be subjected to temperature adjustment, a high-temperature cooling liquid exchanges heat with air through the second heat exchanger, high-temperature air enters the space to be subjected to temperature adjustment to achieve the temperature rise of the space to be subjected to temperature adjustment, and the third heat exchanger enables low-temperature hydrogen to exchange heat with high-temperature cooling liquid, so that temperature rise of hydrogen and cooling of the cooling liquid are achieved so as to guarantee that the fuel cell stack works in the optimal state. According to the fuel cell system, through the first heat exchanger, the second heat exchanger and the third heat exchanger, heating of hydrogen, cooling of cooling liquid and adjustment of the temperature ofthe space to be subjected to temperature adjustment are achieved, so that comprehensive utilization of internal energy of the fuel cell vehicle is achieved.
Owner:TSINGHUA UNIV
Quantitative, high-throughput screening method for protein stability
InactiveUS7148071B2Improve completenessImprove stabilitySamplingDisease diagnosisHigh-Throughput Screening MethodsHigh flux
In proteomic research, it is often necessary to screen a large number of polypeptides for the presence of stable structure. Described herein are methods (referred to as MALDI MS-HX and SUPREX) for measuring the stability of proteins in a rapid, high-throughput fashion. The method employs hydrogen exchange to estimate the stability of quantities of unpurified protein extracts, using matrix-assisted laser desorption / ionization (MALDI) mass spectrometry. A method of quantitatively determining the stability of a test protein under native conditions is disclosed. The method includes the steps (a) providing a test protein; (b) contacting the protein with an exchange buffer comprising a denaturant and deuterium, the exchange buffer having a denaturant concentration; (c) contacting the test protein with a mass spectrometry matrix medium; (d) determining a change in mass of the test protein by mass spectrometry; (e) varying the denaturant concentration of the exchange buffer; (f) repeating steps (a)–(e) a desired number of times; and (g) quantitatively determining protein stability based on the change in mass of the test protein as a function of denaturant concentration, whereby the stability of a test protein under native conditions is quantitatively determined.
Owner:DUKE UNIV
Air pre-cooling compression aero-engine and hypersonic aircraft
ActiveCN106014637BHigh fuel specific impulseImprove economyTurbine/propulsion engine coolingGas turbine plantsCombustion chamberHydrogen exchange
Owner:NAT UNIV OF DEFENSE TECH
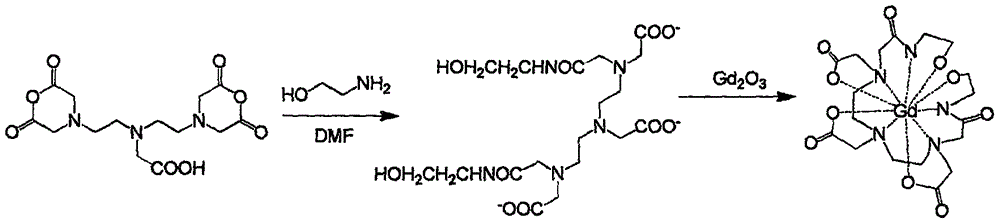
Gadolinium complex used for solution paramagnetic relaxation enhancement probe and synthetic method thereof
InactiveCN105837606AReduce the impactNot easy to interactGroup 3/13 organic compounds without C-metal linkagesSynthesis methodsHydrogen exchange
The invention provides a kind of gadolinium complex Gd-DTPA-BEA used as a solution paramagnetic relaxation enhancement probe, the structural formula is The gadolinium complex has the advantages that it is not easy to interact with the hydrophobic region of the protein, and does not undergo hydrogen exchange, which is beneficial to the accurate analysis of protein dynamics. In addition, the synthesis method of the gadolinium complex is simple in operation and convenient in reaction.
Owner:WUHAN INST OF PHYSICS & MATHEMATICS CHINESE ACADEMY OF SCI
Quantitative, high-throughput screening method for protein stability
InactiveUS7413906B2Improve completenessImprove stabilitySamplingDisease diagnosisHigh-Throughput Screening MethodsHydrogen exchange
In proteomic research, it is often necessary to screen a large number of polypeptides for the presence of stable structure. Described herein are methods (referred to as MALDI MS-HX and SUPREX) for measuring the stability of proteins in a rapid, high-throughput fashion. The method employs hydrogen exchange to estimate the stability of quantities of unpurified protein extracts, using matrix-assisted laser desorption / ionization (MALDI) mass spectrometry. A method of quantitatively determining the stability of a test protein under native conditions is disclosed. The method includes the steps (a) providing a test protein; (b) contacting the protein with an exchange buffer comprising a denaturant and deuterium, the exchange buffer having a denaturant concentration; (c) contacting the test protein with a mass spectrometry matrix medium; (d) determining a change in mass of the test protein by mass spectrometry; (e) varying the denaturant concentration of the exchange buffer; (f) repeating steps (a)-(e) a desired number of times; and (g) quantitatively determining protein stability based on the change in mass of the test protein as a function of denaturant concentration, whereby the stability of a test protein under native conditions is quantitatively determined.
Owner:DUKE UNIV
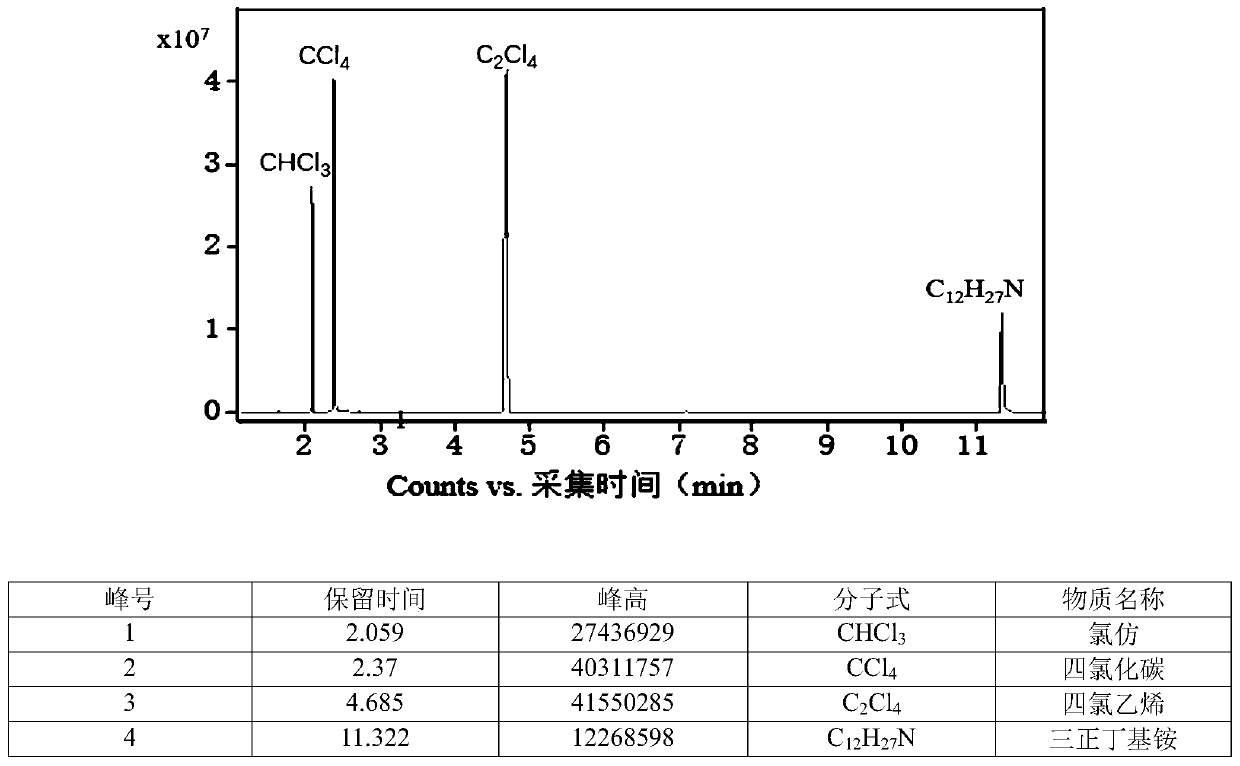
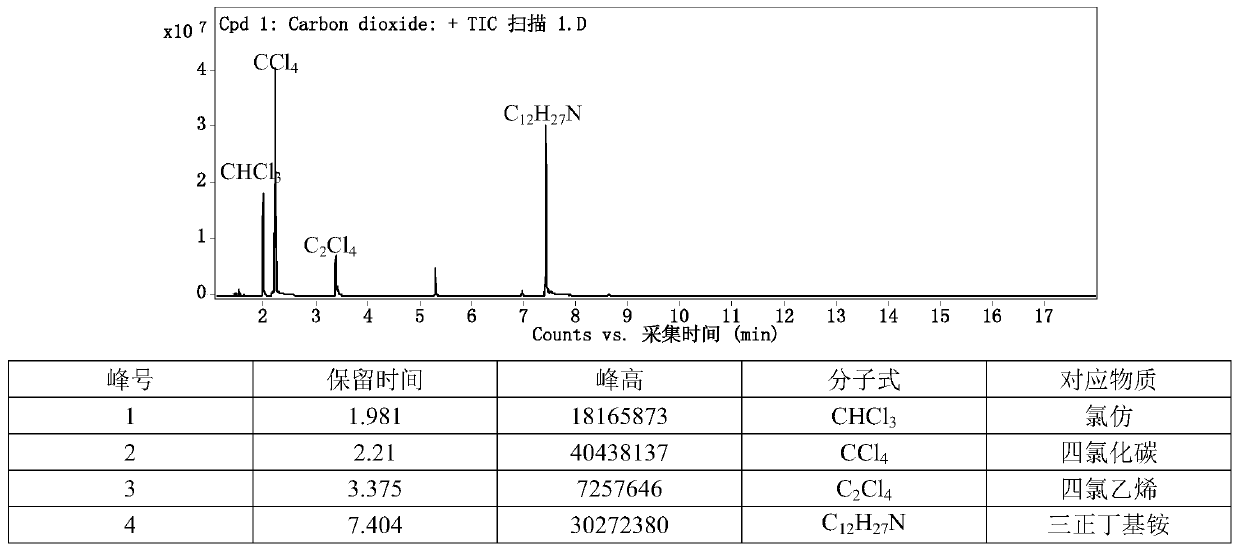
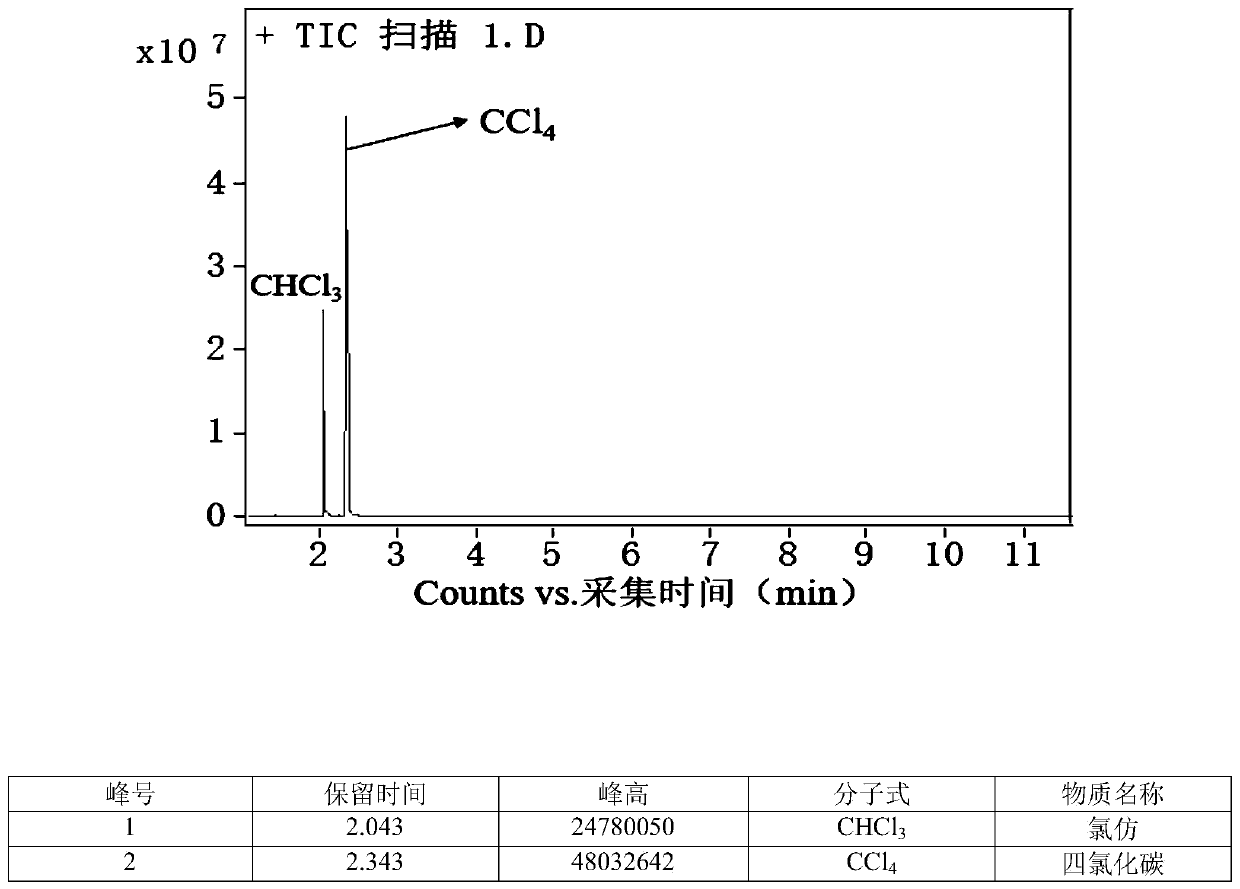
Chlorine-hydrogen exchange method for converting carbon tetrachloride into chloroform
ActiveCN111170825AHigh selectivityEasy to operatePreparation by dehalogenationPtru catalystPotassium hydroxide
The invention provides a chlorine-hydrogen exchange method for converting carbon tetrachloride into chloroform. A conversion reaction can be carried out at normal temperature and normal pressure; a hydrogen donor is trichloroethylene, acetylene, succinimide or other organic matters with active hydrogen, a phase transfer catalyst represented by tetrabutylammonium fluoride is utilized, and alkalineconditions are provided by using potassium hydroxide or potassium carbonate or other substances. On one hand, a noble metal catalyst is not used; on the other hand, reaction conditions are mild, operation is easy and safe, and the method has the advantages of high conversion rate and good selectivity. According to the method, carbon tetrachloride can be converted into chloroform, and meanwhile, the hydrogen donor is converted into a corresponding chlorine substitution product (namely, the hydrogen donors acetylene and trichloroethylene are converted into tetrachloroethylene, and the hydrogen donor succinimide is converted into N-chlorosuccinimide) in a high-selectivity manner, so recycling and high-valued utilization of carbon tetrachloride are realized. The method is suitable for large-scale industrial conversion of carbon tetrachloride, and has great market prospects and high economic values.
Owner:BEIJING UNIV OF CHEM TECH
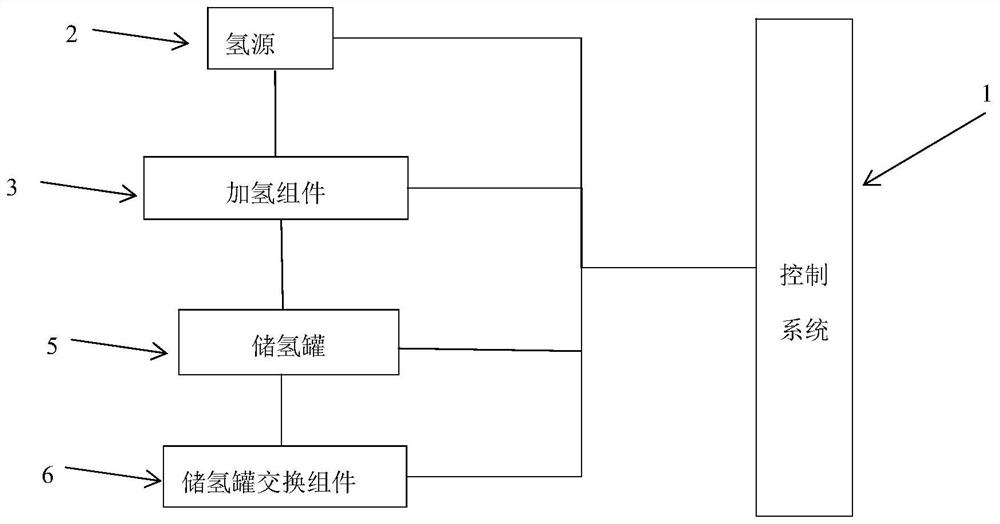
Hydrogen storage tank hydrogenation and hydrogen storage tank exchange all-in-one machine
PendingCN114673926AHydrogenation realizedExchangeGas handling applicationsPipeline systemsThermodynamicsHydrogen exchange
The invention provides a hydrogen storage tank hydrogenation and hydrogen storage tank exchange all-in-one machine, which comprises a hydrogen source, a hydrogenation assembly, a hydrogen storage tank, a hydrogen storage tank exchange assembly and a control system, the hydrogenation assembly is used for adding hydrogen generated by the hydrogen source into the hydrogen storage tank, the hydrogen storage tank exchange assembly is used for exchanging the hydrogen storage tank, and the control system is used for controlling the hydrogen storage tank to exchange the hydrogen. The control system controls the hydrogen source, the hydrogenation assembly and the hydrogen storage tank exchange assembly. The all-in-one machine system has the advantages of being safe, efficient, intelligent, automatic and the like, the whole system is small in size and high in integration, and hydrogen storage tank recycling, hydrogenation and exchange can be safely, reliably and rapidly achieved by placing the hydrogen storage tank in the all-in-one machine.
Owner:JIANGSU JICUI ANTAI CHUANGMING ADVANCED ENERGY MATERIALS RES INST CO LTD
Enhanced methods for crystallographic structure determination employing hydrogen exchange analysis
InactiveUS7363171B2Simple methodHighly varied and highly efficient fragmentationPeptide preparation methodsBiological testingHydrogen exchangeNuMA Protein
The present invention provides methods for crystallographic structure determination employing hydrogen exchange analysis. Hydrogen exchange analysis is used to identify unstructured regions of a protein, and then hydrogen exchange analysis repeated after the protein is admixed with candidate agents and / or conditions that may induce structure in said identified unstructured regions. Agents that induce desired structure in the protein are then employed, admixed with the protein, in co-crystallization studies for structure determination. Hydrogen exchange analysis is performed by determining the quantity of isotope and / or rate of exchange of peptide amide hydrogen(s) with isotope on a labeled protein. Proteins with agent-induced decreases in unstructured regions, and thus improved hydrogen exchange structural maps, are optimal for high quality crystallization and structure determination.
Owner:RGT UNIV OF CALIFORNIA
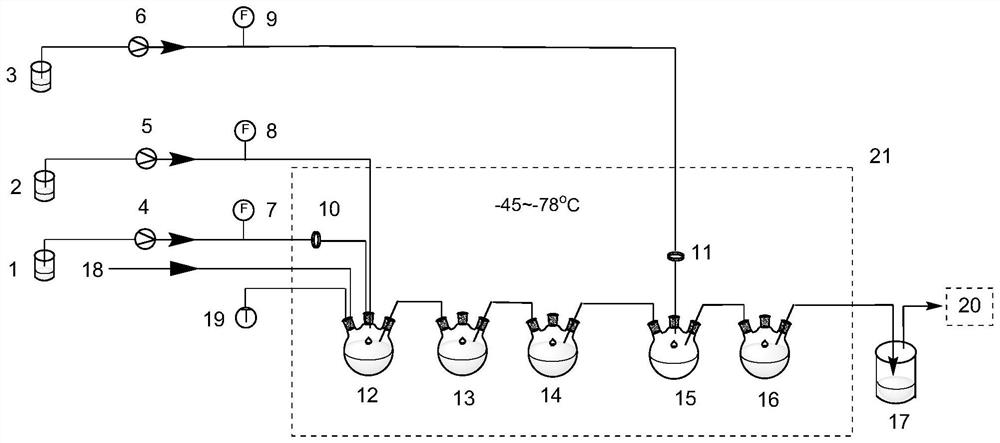
Method for rapidly synthesizing 3-bromo-2-fluorobenzaldehyde based on continuous flow reaction technology
PendingCN113264819AAvoid magnificationReduce the risk factorOrganic compound preparationCarbonyl compound preparation by condensationOrganolithium reagentAldehyde formation
The invention discloses a method for rapidly synthesizing 3-bromine-2-fluorobenzaldehyde based on a continuous flow reaction technology. The method comprises a lithium-hydrogen exchange reaction and a nucleophilic hydroformylation reaction, wherein the lithium-hydrogen exchange reaction comprises the following steps: pumping an o-fluorobromobenzene solution and an organic lithium reagent solution into a lithium-hydrogen exchange continuous reactor according to a certain equivalent proportion; reacting for a certain time at a certain temperature, and carrying out lithium-hydrogen exchange to generate a 3-bromo-2-fluorophenyl lithium intermediate; and the nucleophilic hydroformylation reaction comprises the steps: introducing the active intermediate obtained in the lithium-hydrogen exchange reaction into a hydroformylation continuous reactor, pumping N, N-dimethylformamide in a certain equivalent proportion into the hydroformylation continuous reactor, and reacting at a certain temperature for a certain time to generate a 3-bromo-2-fluorobenzaldehyde product. By applying the continuous flow reaction technology, the traditional kettle type reaction is improved into a continuous process, the amplification effect problem of the traditional kettle type reaction is solved, the danger level of the dangerous reaction of the metal-containing reagent is reduced, the reaction can obtain higher product purity under the controllable continuous condition, and the production efficiency is improved.
Owner:BIRDO (SHANGHAI) PHARM R&D CO LTD
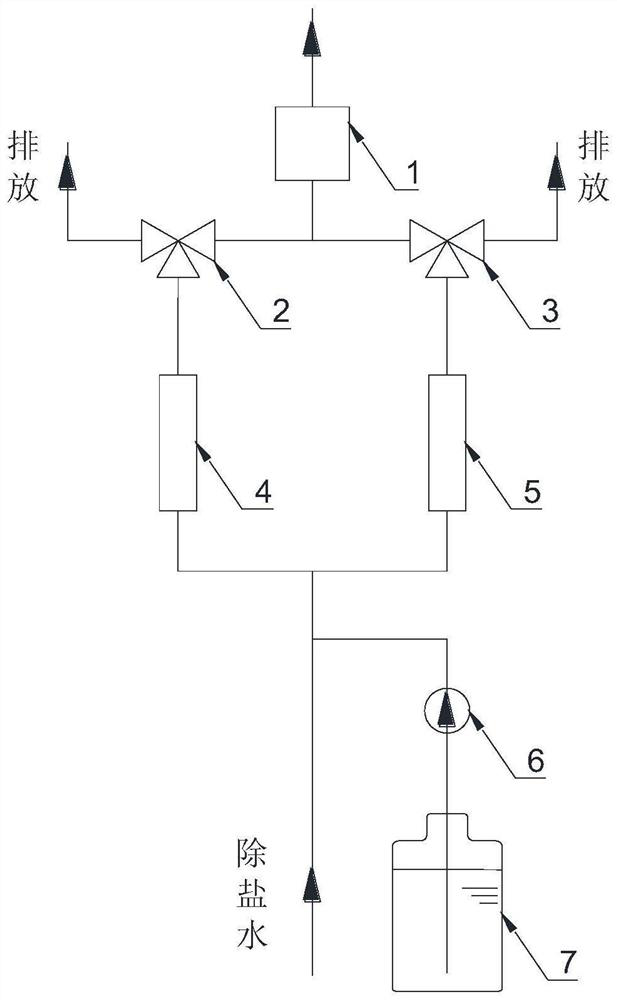
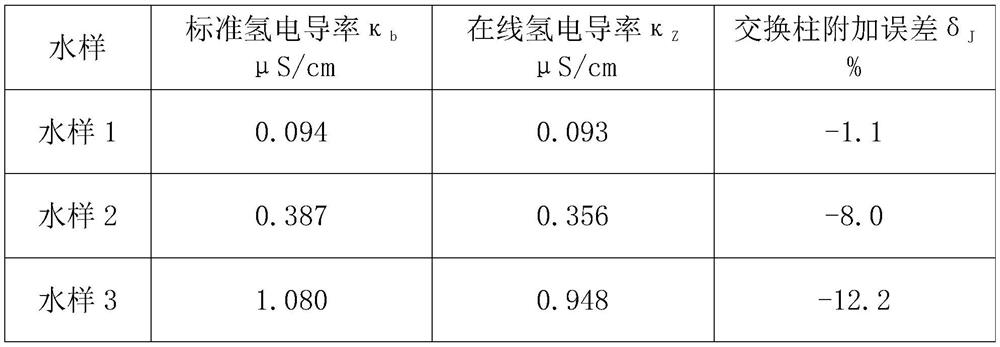
Online hydrogen conductivity meter hydrogen exchange column additional error test system and method
PendingCN114441602AFix measurement inaccuraciesAvoid interferenceMaterial resistanceHydrogen exchangeConductivity
According to the online hydrogen conductivity meter hydrogen exchange column additional error test system and method disclosed by the invention, a solution in a standard solution bottle is added into demineralized water through an adjustable micro pump, and the power of the adjustable micro pump is adjusted for multiple times to obtain a plurality of water samples with different anion concentrations; after a water sample passes through the standard hydrogen exchange column and the detected online hydrogen exchange column at the same time, the conductivity of the water sample passing through the standard hydrogen exchange column and the detected online hydrogen exchange column is measured through the standard conductivity meter, and then the additional error of the detected online hydrogen exchange column is calculated according to the measured conductivity value. And the maximum value of the additional error is taken as the additional error of the detected online hydrogen exchange column, so that the additional error of the hydrogen exchange column of the online hydrogen conductivity meter can be comprehensively and accurately detected.
Owner:大唐秦岭发电有限公司
A kind of synthetic method of toluene-d8
ActiveCN111440041BHigh rate of deuteriumReduce dosageHydrocarbon purification/separationHydrocarbon from halogen organic compoundsPtru catalystHydrogen exchange
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
A method for rapid hydrogen exchange after emergency purging of a bell-type furnace
ActiveCN103451408BReduce oven timeReduce oxidationFurnace typesHeat treatment furnacesHydrogen exchangeSurface oxidation
The invention provides a method for fast exchanging hydrogen after the urgency purging of a bell-type furnace. The method comprises the following steps: manufacturing a simulating heating cover signal plug; programming a fast hydrogen exchange program of an annealing control computer; setting nitrogen purging time and heating cover ignition time to 0, and setting hydrogen exchange time to 12 minutes; controlling furnace atmosphere O2 to be less than 1; when the hydrogen is fast exchanged, firstly disassembling a cooling cover signal plug, connecting the simulating heating cover signal plug, and then starting a heating program to automatically carry out hydrogen exchange according to the set time; and after the hydrogen exchange is finished, disassembling the simulating heating cover signal plug, connecting the cooling cover signal plug, and continuously cooling. According to the method provided by the invention, the integral hydrogen exchange process only needs 30 minutes; the surface oxidation of a steel plate can be effectively reduced, the surface quality of the steel plate can be enhanced, and the second annealing repair of an oxidized steel plate can be avoided; the energy resources are greatly saved, and the production cost is reduced.
Owner:ANGANG STEEL CO LTD
Preparation method and application of a nitrogen-doped carbon-supported low-platinum metal spherical nanoparticle electrocatalyst with uniform particle size
ActiveCN111129513BSimple manufacturing processEase of mass productionMaterial nanotechnologyFinal product manufacturePtru catalystActive agent
Owner:DALIAN UNIV OF TECH
An electrochemical compression heat exchange fresh air system and control method
The invention discloses an electrochemical compression heat exchange fresh air system and a control method thereof, belonging to the field of fresh air systems. The fresh air system includes a fresh air pipeline group and an electrochemical compression refrigeration device. The fresh air pipeline group includes a first fresh air channel and a second fresh air channel; the first metal hydride heat exchanger and the second metal hydride heat exchanger of the electrochemical compression refrigeration device correspond to Set in the first fresh air channel and the second fresh air channel; according to the hydrogen reversing switching signal, control the conduction and air intake of the first fresh air channel or the second fresh air channel to ensure that the fresh air entering the room is always cold or hot. It not only realizes the adjustment of indoor temperature, but also realizes the introduction of indoor fresh air, reduces the operating noise, does not require refrigerant, is more green and environmentally friendly, and brings a new all-round comfortable experience of temperature adjustment and air quality improvement to the human body. Control method: receiving the hydrogen reversing switching signal sent by the electrochemical compression refrigeration device.
Owner:QINGDAO HAIER SMART TECH R & D CO LTD +2
A method for improving the hydrogen-deuterium atom exchange efficiency of protein in deuterated reagent
The invention provides a method for improving hydrogen and deuterium atomic exchanging efficiency of protein in nuclear magnetism reagent by pressurizing inert gas and radiating isotopes under an anaerobic condition. The to-be-detected protein is dissolved in the nuclear magnetism reagent, and inert gas is introduced into a solution system so as to increase the pressure to 0.15MPa to 1.0MPa. The radioactive source selects radioactive isotopes such as cobalt-60 and caesium-137, and the dosage is 5kGy to 500kGy. The detected protein basically contains all protein types, and the exchanging rate is detected in a mass-spectrography.
Owner:贾明宏
Ruthenium-Catalyzed Ortho-Meta Selective Hydrogen-Deuterium Exchange Reaction of Benzene Ring
Owner:RENMIN UNIVERSITY OF CHINA
A kind of method for preparing methyl 4-methoxyvalerate by gamma-valerolactone
ActiveCN104341294BImprove performanceGreat application potentialMolecular sieve catalystsPreparation by ester-hydroxy reactionMolecular sieveDistillation
The invention discloses a method for preparing methyl 4-methoxy valerate from gamma-valerolactone and relates to methyl 4-methoxy valerate. The invention provides a low-cost and environment-friendly method for preparing methyl 4-methoxy valerate from gamma-valerolactone. The method comprises the following steps: (1) adding a hydrogen-exchange ultrastable Y-type molecular sieve and CaCO3 as catalysts into a mixed solution of gamma-valerolactone and methanol and reacting under an inert gas atmosphere to obtain a solid-liquid mixture; and (2) carrying out reduced pressure suction filtration on the solid-liquid mixture obtained in the step (1) to obtain a mixed solution and carrying out reduced pressure distillation on the obtained mixed solution to obtain methyl 4-methoxy valerate. Methyl 4-methoxy valerate is synthesized in a high yield from gamma-valerolactone and methanol by synergetic catalysis of the hydrogen-exchange ultrastable Y-type molecular sieve and CaCO3 for the first time and the method provides an entirely new way to biomass synthesis of high value-added chemicals.
Owner:XIAMEN UNIV
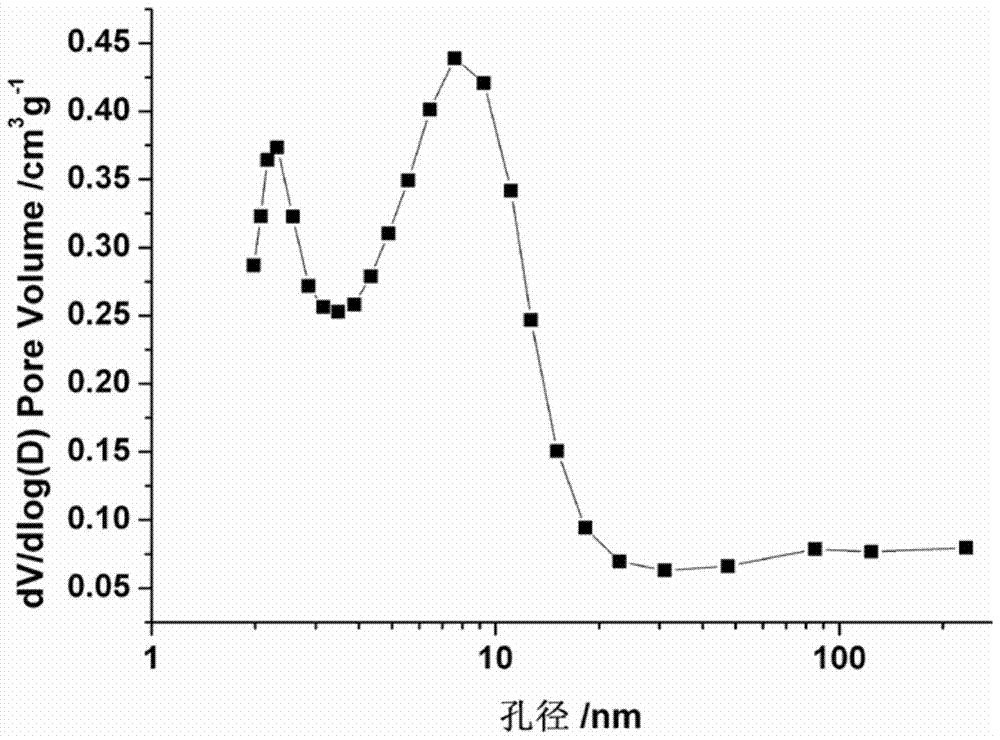
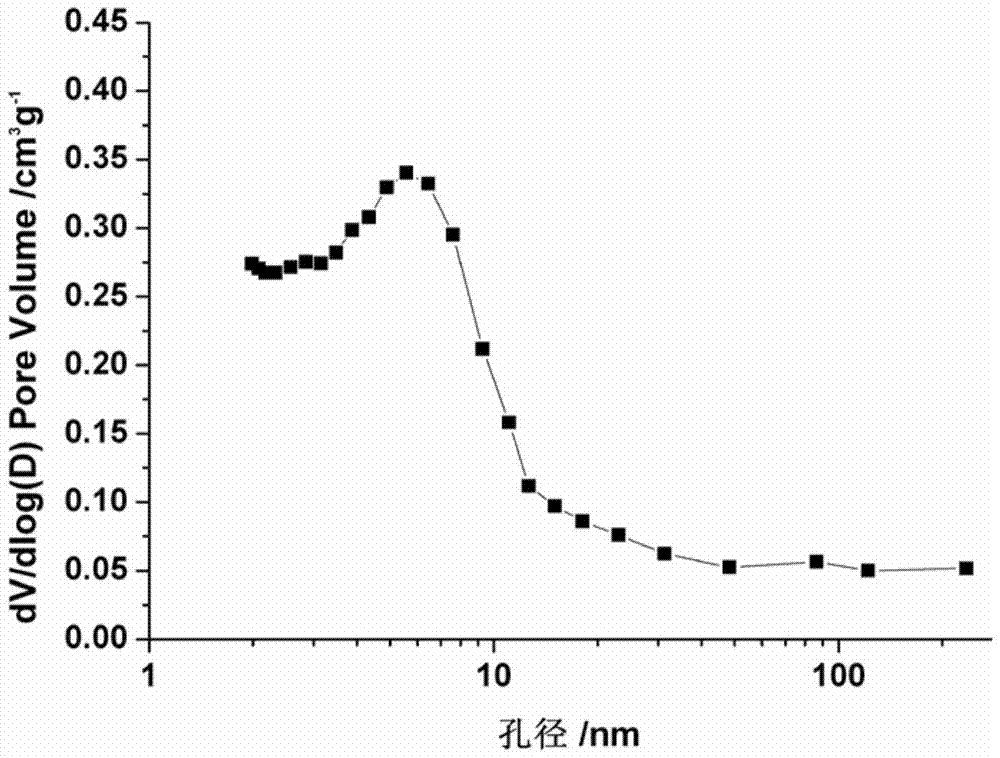
A method for treating molecular sieves with fluorine-containing alkaline medium
ActiveCN104724722BAdjustable secondary hole structureSimple stepsMordenite aluminosilicate zeolitePentasil aluminosilicate zeoliteMolecular sieveHydrogen exchange
The invention discloses a method for treating molecular sieves with a fluorine-containing alkaline medium, which belongs to the fields of molecular sieve modification, adsorption material preparation and catalyst preparation. The method is to introduce fluorine element into the molecular sieve alkali treatment medium in a certain way, then heat treatment, filter and wash to neutrality, and after drying, ion exchange and roasting are carried out to exchange into hydrogen molecular sieve. This method is simple in operation and low in cost. The micropores of the modified molecular sieve material are maintained well, and a rich secondary pore structure is produced, while the acidity is maintained well.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com