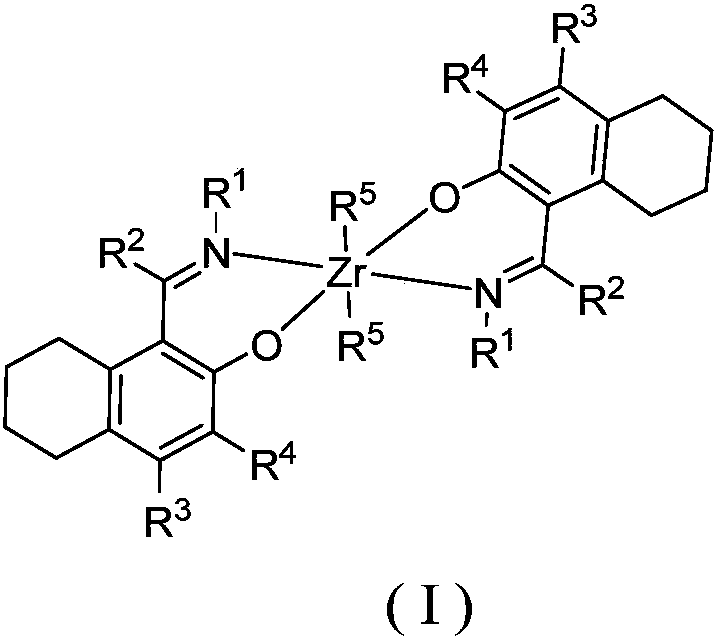
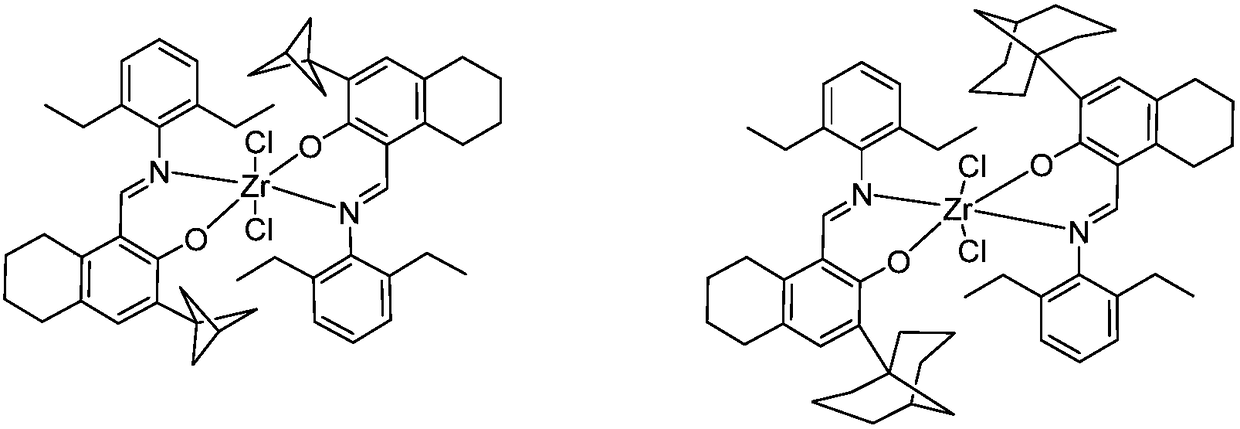
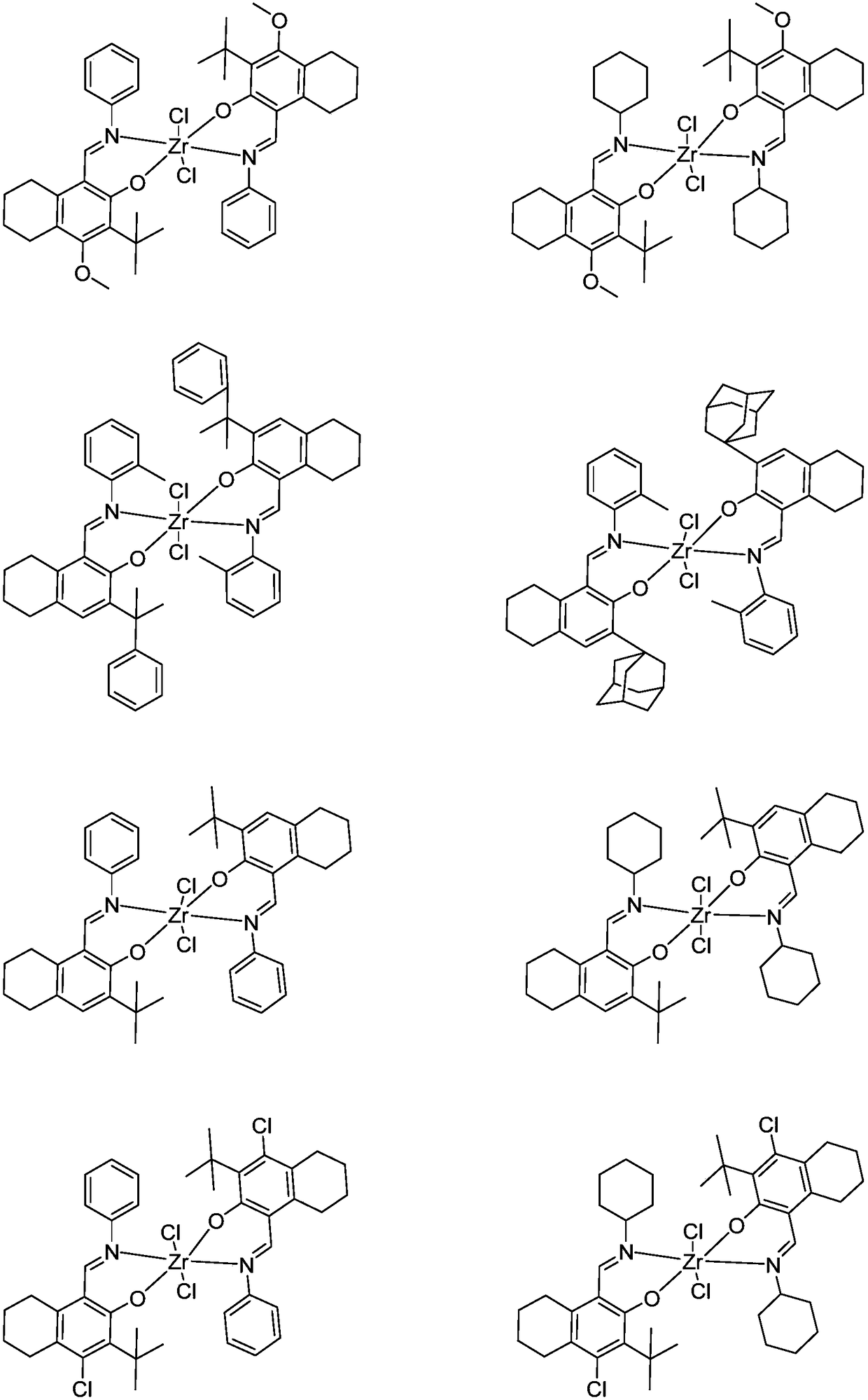
Zirconium tetralylmethoxyl imine complex and preparation method and application thereof
A technology of zirconium tetrahydronaphthyloxyimine complexes, which is applied in the field of zirconium tetrahydronaphthyloxyimine complexes and their preparation, can solve the problems of deactivation, wide molecular weight distribution, and low electron density, and achieve a reduction in Effects of differences in reactivity ratio, increased charge density, and narrow molecular weight distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Synthesis of Ligand L1
[0037] Add intermediate M1 (5.25g, 20mmol), absolute ethanol (50mL) and 1.0g molecular sieves to the 100mL reaction flask successively Stir at room temperature for 5 minutes, add aniline (1.86 g, 20 mmol), and react under reflux for 16 hours. The reaction liquid was filtered, and the solvent was removed from the filtrate under reduced pressure, and the product L1 (4.06 g, yield: 60.1%) was obtained by recrystallization from petroleum ether. The reaction process is shown in the following formula:
[0038]
[0039] 1 H NMR (CDCl 3 ,400MHz): δ8.68(s,1H,CH=N),7.47(s,4H, 4 J=2.2Hz,ArH),7.07(s,1H, 4 J=2.2Hz, ArH), 5.84(s, 1H, OH), 3.78(s, 3H, OCH 3 ),2.92(t,2H, 3 J=7.2Hz, NCH 2 ),2.74(t,2H, 3 J=7.2Hz, NCH 2 ),1.71(t,4H, 3 J=7.2Hz, NCH 2 ),1.35(s,9H,C(CH 3 ) 3 ). 13 C NMR (CDCl 3 for C 22 h 27 NO 2 H, 8.06; N, 4.15. Found: C, 78.01; H, 8.02; N, 4.07%.
Embodiment 2
[0041] Synthesis of Ligand L2
[0042] Add intermediate M1 (5.25g, 20mmol), absolute ethanol (50mL) and 1.0g molecular sieves to the 100mL reaction flask successively . Stir at room temperature for 5 minutes, add cyclohexylamine (1.98 g, 20 mmol), and react under reflux for 16 hours. The reaction liquid was filtered, and the solvent was removed from the filtrate under reduced pressure, and the product L2 (5.50 g, yield: 80.1%) was obtained by recrystallization from petroleum ether. The reaction process is shown in the following formula:
[0043]
[0044] 1 H NMR (CDCl 3 ,400MHz): δ8.61(s,1H,CH=N),5.84(s,1H,OH),3.78(s,3H,OCH 3 ),2.92(t,2H, 3 J=7.2Hz, NCH 2 ),2.74(t,2H, 3 J=7.2Hz, NCH 2 ),2.19(t,4H, 3 J=7.2Hz, NCH 2 ),1.71(t,4H, 3 J=7.2Hz, NCH 2 ),1.66(t,4H, 3 J=7.2Hz, NCH 2 ),1.45(t,2H, 3 J=7.2Hz, NCH 2 ),1.35(s,9H,C(CH 3 ) 3 ). 13 C NMR (CDCl 3 for C 22 h 33 NO 2 H, 9.68; N, 4.08. Found: C, 75.21; H, 9.32; N, 4.07%.
Embodiment 3
[0046] Synthesis of Ligand L3
[0047] Add intermediate M3 (6.77g, 20mmol), absolute ethanol (50mL) and 1.0g molecular sieves to the 100mL reaction flask successively Stir at room temperature for 5 minutes, add aniline (1.98 g, 20 mmol), and react under reflux for 16 hours. The reaction liquid was filtered, and the solvent was removed from the filtrate under reduced pressure, and the product L3 (5.70 g, yield: 69.0%) was obtained by recrystallization from petroleum ether. The reaction process is shown in the following formula:
[0048]
[0049] 1 H NMR (CDCl 3 ,400MHz):δ7.34-7.13(m,8H,ArH),6.93(d,2H, 4 J=2.4Hz, ArH), 3.78(s, 6H, OCH 3 ),2.92-2.74(t, 3 J=7.2Hz, 4H, NCH 2 ),1.81(s,3H,NCCH 3 ),1.69(s,6H,CPh(CH 3 ) 2 ). 13 C NMR (CDCl 3 for C 28 h 31 NO 2 H, 7.56; N, 3.39. Found: C, 81.02; H, 7.35; N, 3.26%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com