Chlorine-hydrogen exchange method for converting carbon tetrachloride into chloroform
A technology of carbon tetrachloride and chloroform, applied in the field of chlorine-hydrogen exchange method for converting carbon tetrachloride into chloroform, achieving the effect of high selectivity and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
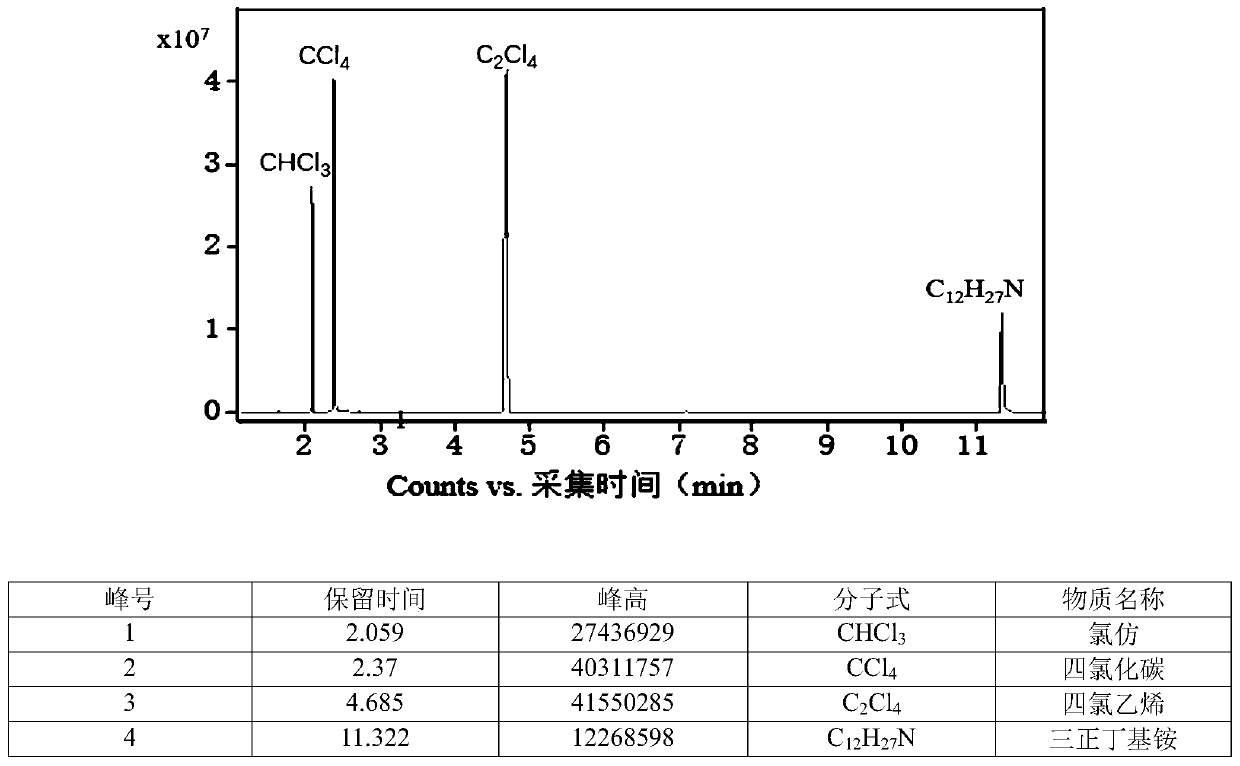
[0030]Take 1 part of trichloroethylene, 6 parts of carbon tetrachloride, 0.1 part of TBAF, and 1 part of potassium carbonate, put it in a round-bottom flask, and stir at room temperature for 1 h. The liquid layer was taken for gas chromatographic analysis, and the conversion rate of trichloroethylene and the yield of chloroform were calculated. At this point, the conversion of trichloroethylene reached 100%, and the selectivity to chloroform was 95%.
Embodiment 2
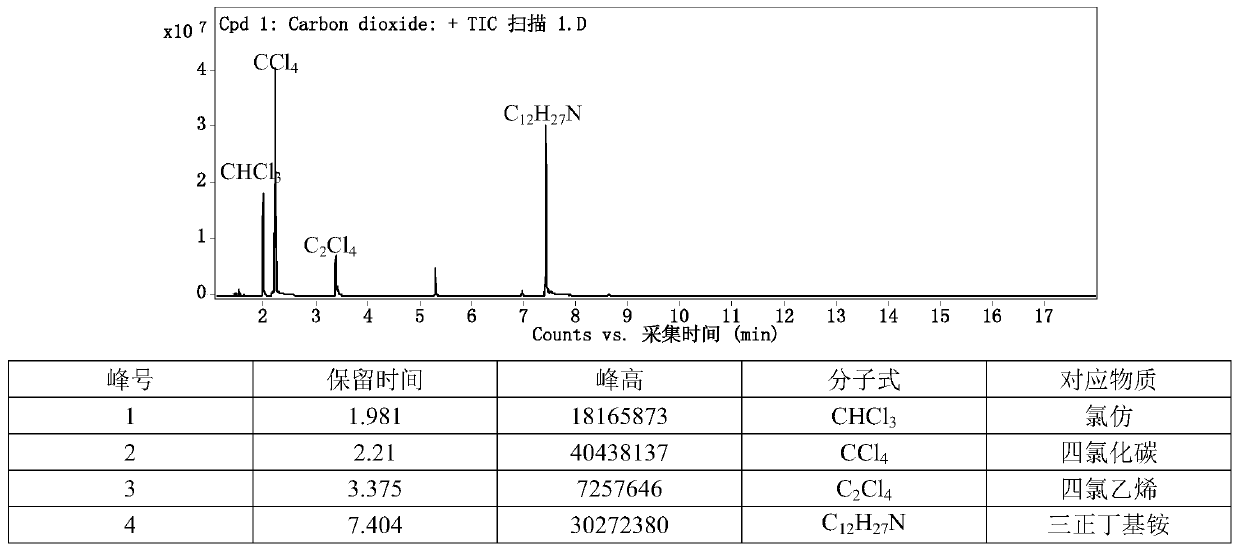
[0032] Take 1 part of trichloroethylene, 6 parts of carbon tetrachloride, 0.1 part of TBAF, and 1 part of sodium metaaluminate, put it in a round-bottomed flask, and stir at 20° C. for 5 h. The liquid layer was taken and analyzed by gas chromatography, and the conversion of trichloroethylene reached 100%, and the selectivity of chloroform reached 90%.
Embodiment 3
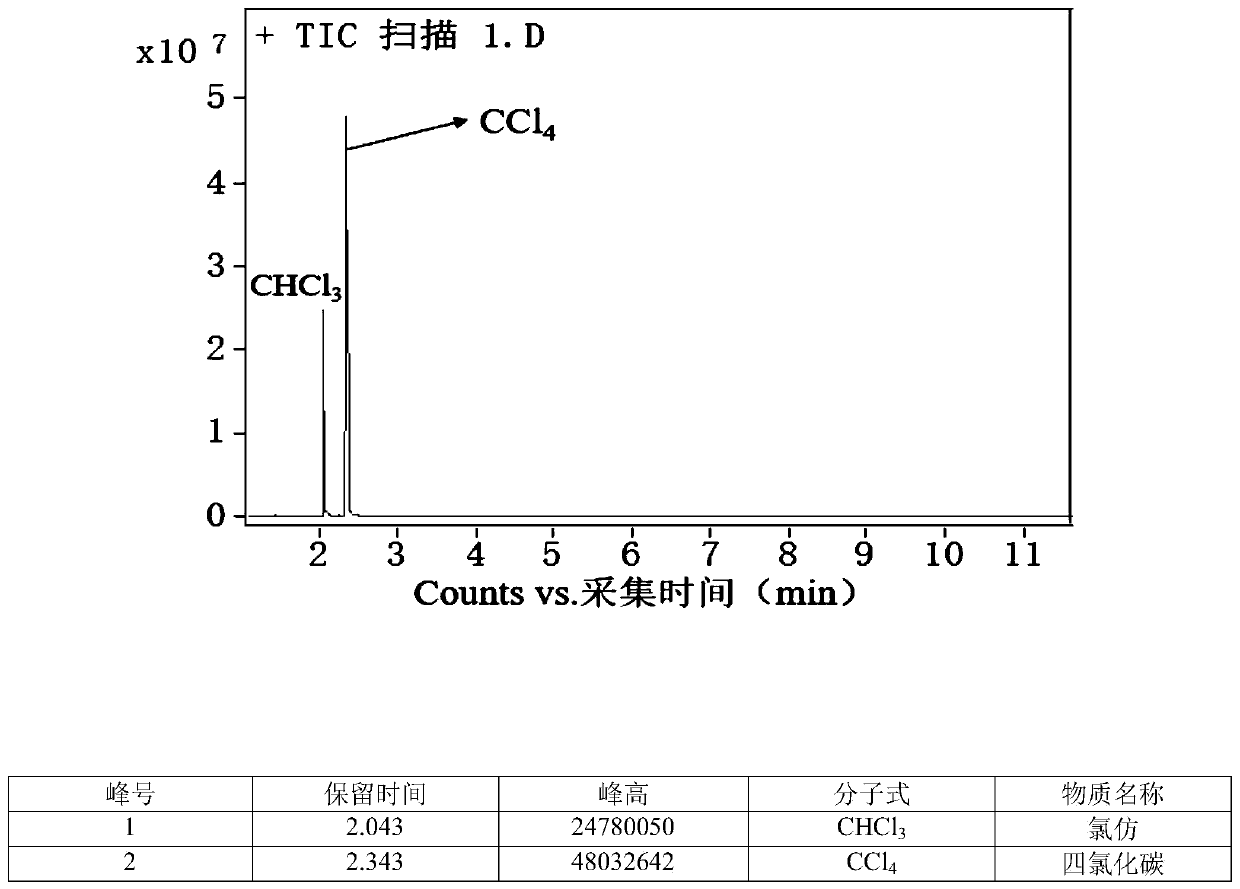
[0034] Take 1 part of trichloroethylene, 6 parts of carbon tetrachloride, 0.1 part of TBAF, and 1 part of potassium hydroxide, put them in a round-bottomed flask, and stir at 20° C. for 0.2 h. The liquid layer was taken and analyzed by gas chromatography, and the conversion of trichloroethylene reached 100%, and the selectivity of chloroform reached 92%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com