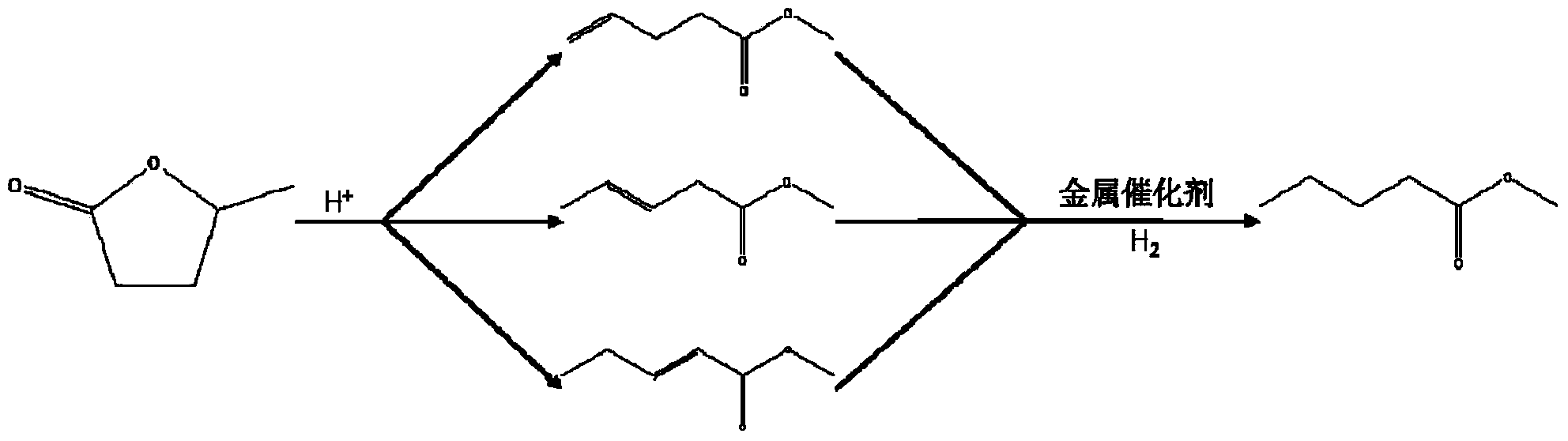
Method for preparing methyl 4-methoxy valerate from gamma-valerolactone
A technology of methyl methoxyvalerate and valerolactone, which is applied in the preparation of ester group and hydroxyl reaction, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problem of lack of material yield, properties and uses attention and other issues to achieve the effect of large application potential and good economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
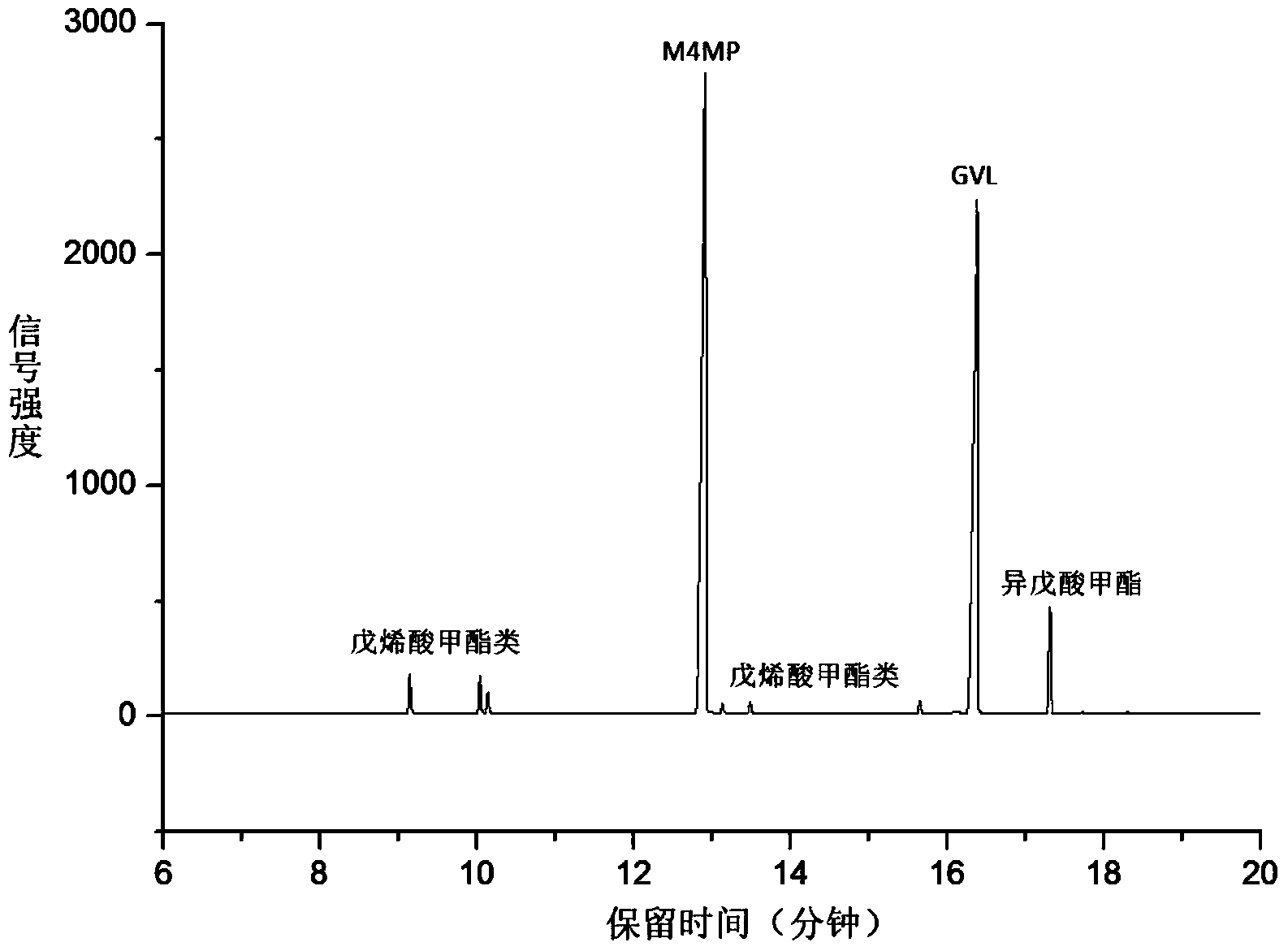
[0018] Add 0.5g hydrogen-exchanged ultra-stable Y-type molecular sieve and 0.05g CaCO to the mixture of 10mL γ-valerolactone and 20mL methanol 3 As a catalyst, it was reacted at 250°C under a hydrogen atmosphere for 4 hours, and then filtered under reduced pressure to obtain a colorless transparent liquid. At this time, the conversion rate of γ-valerolactone was 63%, the selectivity to methyl 4-methoxyvalerate was 81%, and the selectivity to pent(en) esters was 12%.
Embodiment 2
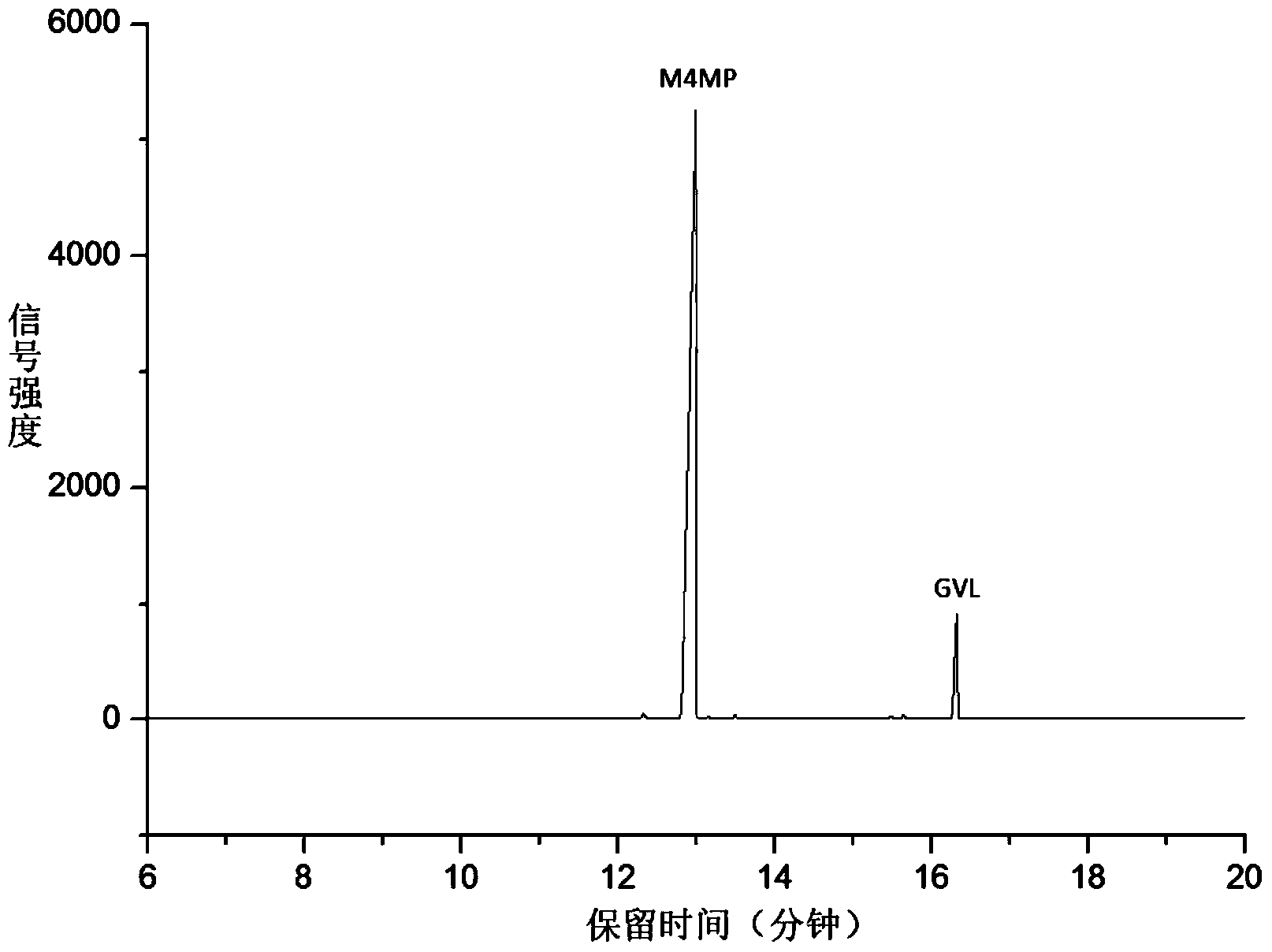
[0020] Add 0.5g hydrogen-exchanged ultra-stable Y-type molecular sieve and 0.1g CaCO to the mixture of 5mL γ-valerolactone and 25mL methanol 3 As a catalyst, it was reacted at 250°C under a hydrogen atmosphere for 4 hours, and then filtered under reduced pressure to obtain a colorless transparent liquid. Now the gamma-valerolactone conversion rate is 65%, and the selectivity to methyl 4-methoxyvalerate is 92%, and the selectivity to pent(en) esters is 8% (such as figure 1 ).
Embodiment 3
[0022] Add 0.5g hydrogen-exchanged ultra-stable Y-type molecular sieve and 0.05g CaCO to the mixture of 10mL γ-valerolactone and 20mL methanol 3 As a catalyst, it was reacted at 250°C under a hydrogen atmosphere for 1 h, and then filtered under reduced pressure to obtain a colorless transparent liquid. At this time, the conversion rate of γ-valerolactone was 39%, the selectivity to methyl 4-methoxyvalerate was 77%, and the selectivity to pent(en) esters was 23%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com