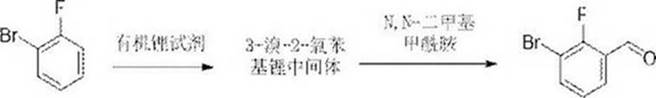Method for rapidly synthesizing 3-bromo-2-fluorobenzaldehyde based on continuous flow reaction technology
A technology of fluorobenzaldehyde and a synthesis method, which is applied in the field of 3-bromo-2-fluorobenzaldehyde synthesis, can solve the problems of increased side reactions, increased time consumption, obvious amplification effect, etc., achieves low cost, solved amplification effect, reduced The effect of the risk factor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
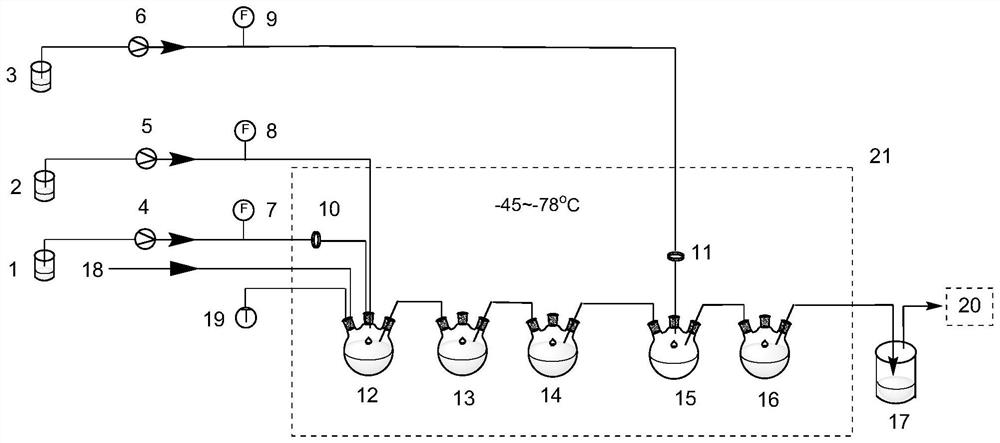
[0026] Embodiment 1: as figure 1 As shown, the THF solution of o-fluorobromobenzene is put into the storage tank 1, the THF solution of LDA is put into the storage tank 2, the temperature control device 21 sets the temperature to be -80°C, the THF solution of o-fluorobromobenzene and LDA The solution is pumped into the first-stage lithium-hydrogen exchange continuous reactor 12 by the first metering pump 4 and the second metering pump 5 respectively, and reacted for 8 minutes, and is extended for 30 minutes through the second-stage and third-stage lithium-hydrogen exchange continuous reactors 13 and 14 In the reaction time, the molar equivalent ratio of o-fluorobromobenzene and LDA is 1:1.5, and the reaction generates 3-bromo-2-fluorophenyllithium intermediate; the active intermediate is passed into the first-level formylation continuous reactor 15, Simultaneously, the THF solution of N,N-dimethylformamide is pumped into reactor 15 to carry out nucleophilic aldylation reaction...
Embodiment 2
[0027] Embodiment 2: The specific preparation process is the same as that in Embodiment 1. The temperature control device 21 is set at -60° C., and the organic phase of the reaction solution is diluted and filtered, and HPLC analysis shows that the concentration of 3-bromo-2-fluorobenzaldehyde in the reaction solution is The conversion rate was 68.78%.
Embodiment 3
[0028] Example 3: The specific preparation process is the same as in Example 1, the molar equivalent ratio of o-fluorobromobenzene and LDA is adjusted to 1:1.7, and the organic phase of the reaction solution is diluted and filtered, and HPLC analysis shows that 3-bromo-2 in the reaction solution -The conversion rate of fluorobenzaldehyde is 70.84%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com