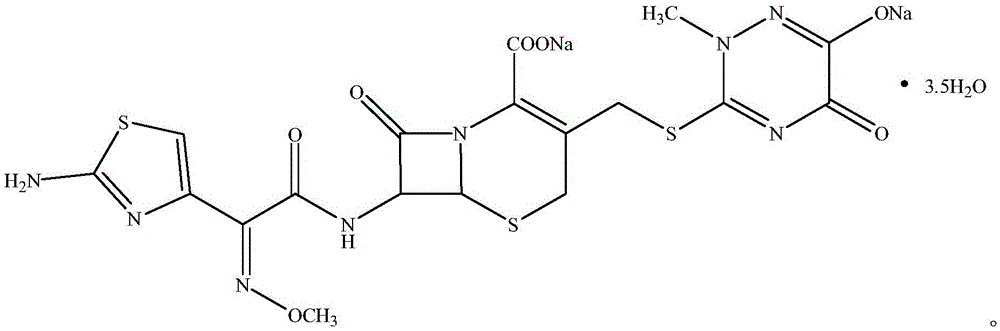
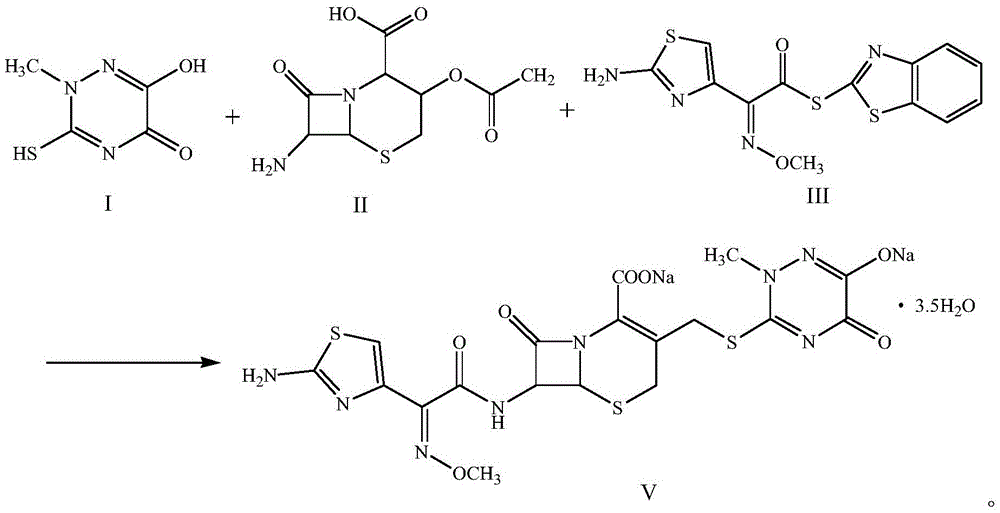
Method for synthesizing ceftriaxone sodium
A technology of ceftriaxone sodium and a compound, applied in the field of medicine, can solve problems such as affecting the production efficiency of manufacturers, and achieve the effects of avoiding amplification effect and simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Add 15.9g of compound I (0.1mol, M.W.159) and 28.49g of compound II (0.11mol, M.W.259) into a reaction flask containing 3.18g of dimethyl carbonate, stir and react at 30°C for 1 hour, then add 38.5g Compound III (0.11mol, M.W.350), add 3.18g PEG-800 while stirring, add 0.975g triethylamine after stirring for 10 minutes, stir and react at 8°C for 3 hours, then add dropwise 5w.t.% sodium hydroxide solution to When pH=7, excess acetone was added, white crystals were precipitated, and dried under vacuum at 20°C to obtain 65.6 g of compound V (M.W.661), with a yield of 99.2%, a purity of over 99.99%, and a total impurity of less than 0.01%.
Embodiment 2
[0025] Add 15.9g of compound I (0.1mol, M.W.159) and 31.08g of compound II (0.12mol, M.W.259) into a reaction flask containing 4.77g of dimethyl carbonate, stir and react at 40°C for 2 hours, then add 42g of compound Ⅲ (0.12mol, M.W.350), add 4.77g PEG-800 while stirring, add 1.59g triethylamine after stirring for 20 minutes, stir and react at 10°C for 4 hours, then add dropwise 5w.t.% sodium hydroxide solution to pH =7, adding excess acetone, white crystals were precipitated, and dried under vacuum at 30°C to obtain 65.6 g of compound V (M.W.661), with a yield of 99.2%, a purity of over 99.99%, and a total impurity of less than 0.01%.
Embodiment 3
[0027] Add 15.9g of compound I (0.1mol, M.W.159) and 28.49g of compound II (0.11mol, M.W.259) into a reaction flask containing 3.18g of dimethyl carbonate, stir and react at 35°C for 1 hour, then add 38.5g Compound III (0.11mol, M.W.350), add 3.18g PEG-800 while stirring, add 0.975g triethylamine after stirring for 10 minutes, stir and react at 10°C for 4 hours, then add dropwise 5w.t.% sodium hydroxide solution to When pH=7, excess acetone was added, and white crystals were precipitated, and vacuum-dried at 30°C to obtain 65.8 g of compound V (M.W.661), with a yield of 99.5%, a purity of over 99.99%, and a total impurity of less than 0.01%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com