Preparation method of deuterated alcohol or deuterated amine compounds
A compound and alcohol technology, applied in the preparation of organic compounds, amino compounds, hydroxyl compounds, etc., can solve the problems of dangerous reaction process, harsh reaction conditions, poor selectivity, etc., to reduce the risk, mild reaction conditions, A stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] Prepare benzhydryl alcohol (2a), structural formula is as follows:
[0101]
[0102] The reaction equation is as follows:
[0103]
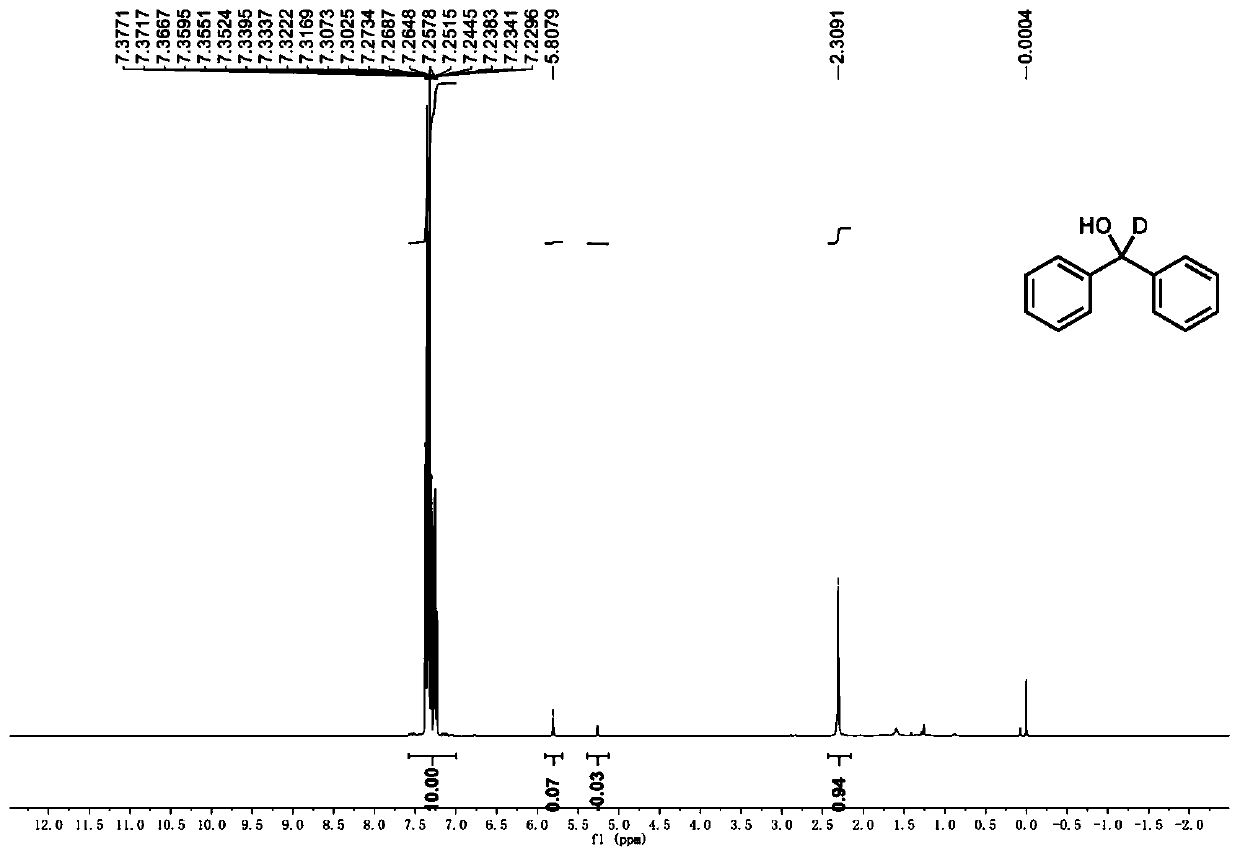
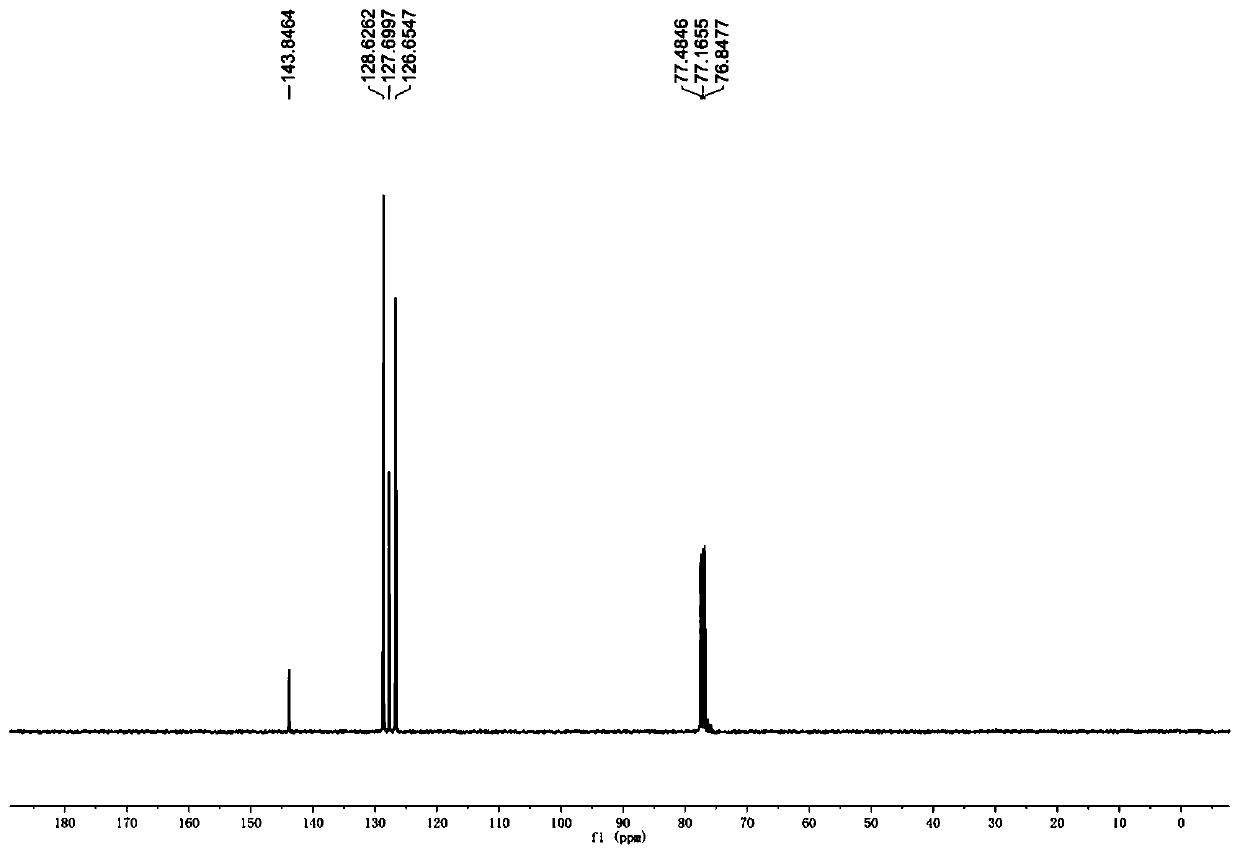
[0104] According to the typical experimental procedure, with benzophenone (1a) (0.0364g, 0.2mmol), catalyst Ir-9 (0.9mg, 0.5mol%), N,N-dicyclohexylmethylamine (86μL, 0.4mmol), Li 2 CO 3 (8.9mg, 0.12mmol), heavy water (36μL, 2mmol), dissolved in 3mL ultra-dry acetonitrile for reaction. After the reaction, the crude product was purified by column chromatography (eluted with PE / EA=100 / 1~50 / 1) to obtain the target product (27mg, yield: 70%, 93% deuteration rate), the product It is a white solid. 1 HNMR (400MHz, CDCl 3 ):δ7.40–7.29 (m,8H), 7.29–7.25(m,2H), 2.33(s,1H). 13 CNMR (101MHz, CDCl 3 ): δ132.91– 116.81(m), 82.61–63.41(m).
Embodiment 2
[0106] Prepare 4-methyl-benzhydryl alcohol (2b), the structural formula is as follows:
[0107]
[0108] The reaction equation is as follows:
[0109]
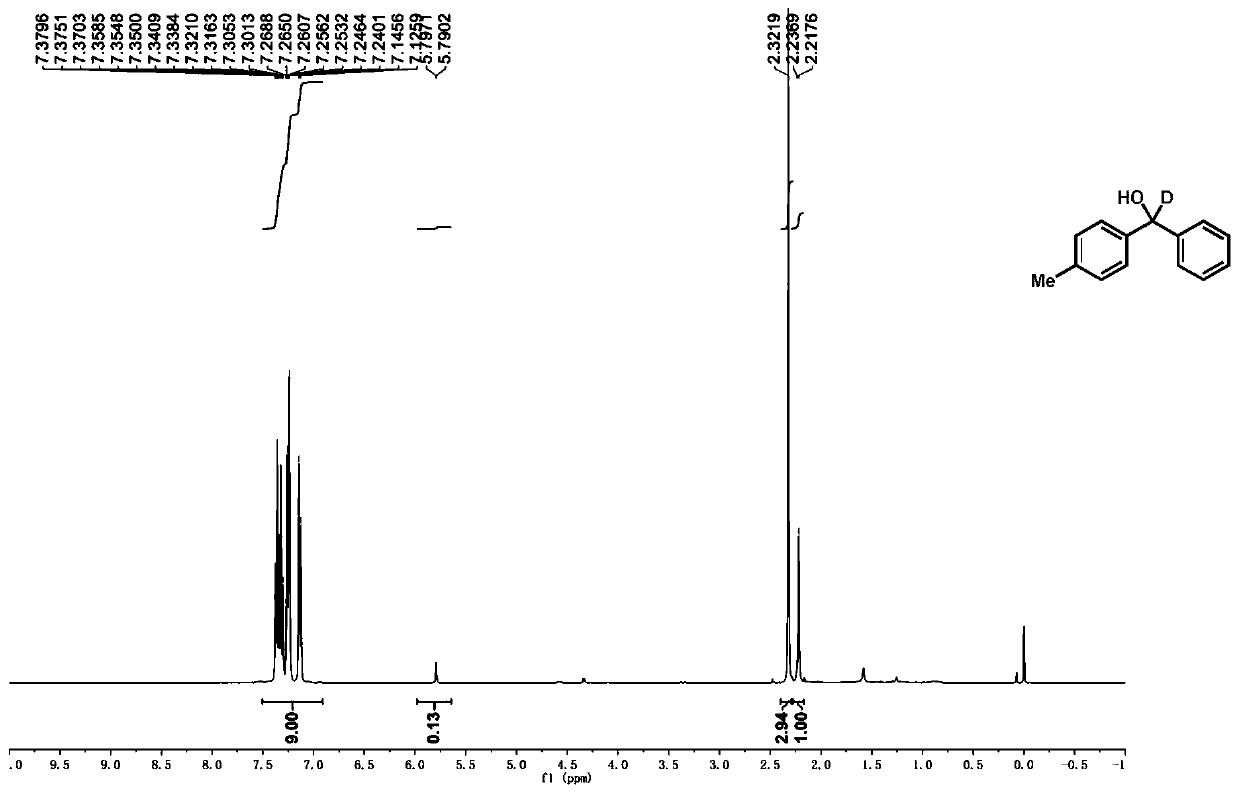
[0110]According to the typical experimental procedure, with 4-methyl-benzophenone (1b) (0.0398g, 0.2mmol), catalyst Ir-7 (0.9mg, 0.5mol%), N,N-dicyclohexylmethylamine (86μL , 0.4mmol), Li 2 CO 3 (8.9mg, 0.12mmol), D 2 O (36 μL, 2 mmol), dissolved in 3 mL ultra-dry acetonitrile for reaction. After the reaction, the crude product was purified by column chromatography (eluted with PE / EA=100 / 1~50 / 1) to obtain the target product (27.9 mg, yield: 70%, 87% deuteration rate), the The product is a white solid. 1 H NMR (400MHz, CDCl 3 ):δ7.56–6.75(m,9H),2.32(s,3H),2.22(s,1H). 13 C NMR (101MHz, CDCl 3 ): δ143.98, 140.99, 137.40, 129.30, 128.57, 127.57, 126.61, 126.54, 76.19, 75.98, 75.76, 75.54, 21.24.
Embodiment 3
[0112] Prepare 2-chloro-benzhydryl alcohol (2c), the structural formula is as follows:
[0113]
[0114] The reaction equation is as follows:
[0115]
[0116] According to the typical experimental procedure, with 2-chloro-benzophenone (1c) (0.0433g, 0.2mmol), catalyst Ir-5 (0.9mg, 0.5mol%), N,N-dicyclohexylmethylamine (86μL, 0.4mmol), Li 2 CO 3 (8.9mg, 0.12mmol), D 2 O (36 μL, 2 mmol), dissolved in 3 mL ultra-dry acetonitrile for reaction. After the reaction, the crude product was purified by column chromatography (eluted with PE / EA=100 / 1~50 / 1) to obtain the target product (40.7 mg, yield: 94%, 89% deuteration rate), the The product is a white solid. 1 H NMR (400MHz, CDCl 3 ):δ7.86–6.77(m,9H),2.41(s,1H). 13 C NMR (101MHz, CDCl 3 ): δ142.24, 141.05, 141.00, 132.58, 129.65, 128.89, 128.60, 128.11, 127.91, 127.24, 127.04, 127.02, 72.78, 72.63, 72.40, 72.18.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com