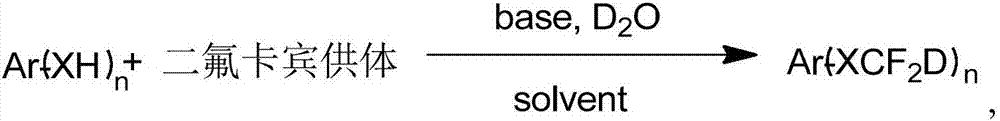
Synthesis method of difluorodeuteromethoxy(thio) function group-containing aromatic compound
A synthesis method and technology of functional groups, applied in the field of deuterium-containing compound synthesis, can solve the problems of inappropriate synthesis of aromatic compounds, inability to meet the purity of deuterated drugs, low deuteration rate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050]
[0051] The compound 2-naphthol (144mg, 1mmol) was dissolved in 4.0ml of dry (metal sodium dehydrated) 1,4-dioxane, under the protection of argon, the temperature was cooled to 15°C in an ice-water bath, and added in batches NaH (60%, 400mg, 10mmol), ensure that the temperature of the reaction system is lower than 25°C during the addition process, then react for 0.5h, and then slowly add heavy water 1ml (50mmol), and ensure that the temperature of the reaction system is lower than 40°C during the dropwise addition. After the dropwise addition, react for 0.5h, add diethyl bromodifluoromethylphosphate (534mg, 2mmol) dropwise, keep the temperature of the reaction system lower than 30°C during the dropwise addition, and raise it to room temperature for 0.5h. After the reaction, 20ml of ether was added, the organic phase was separated from the water phase, the organic phase was washed with saturated ammonium chloride solution (20ml×3), the organic phase was dried with anh...
Embodiment 2- Embodiment 9
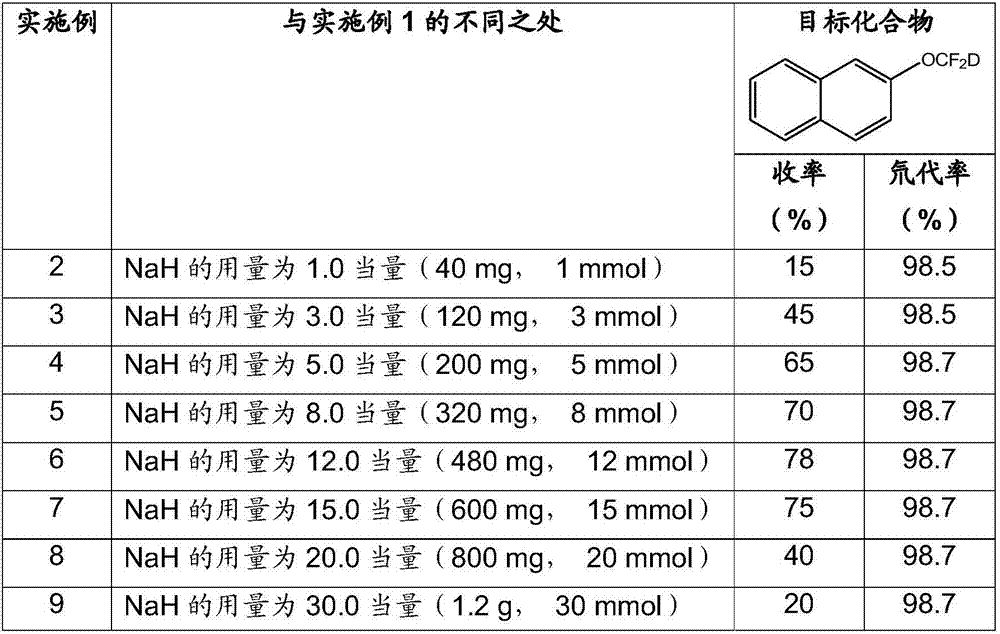
[0057] The following are examples 2-9, the other conditions of the reaction are the same as in Example 1, the only difference is that the amount of alkali NaH is adjusted, and the influence on the yield of the target compound and the deuteration rate under the conditions of different alkali amounts is investigated.
[0058]
[0059] As can be seen from the data in the table above, the amount of alkali has a greater impact on the yield, and at 8-15 equivalents, the yield is higher; but the amount of alkali has little effect on the deuterium substitution rate, and with the adjustment of the amount of alkali, deuterium The generation rate fluctuates very little, and the deuterium rate is ≥98.5%.
Embodiment 10- Embodiment 16
[0061] The following are examples 10-16, the other conditions of the reaction are the same as in Example 1, the difference is only that the amount of heavy water is adjusted, and the influence on the yield of the target compound and the deuterium rate under the conditions of different amounts of heavy water is investigated.
[0062]
[0063] As can be seen from the data in the above table, the amount of heavy water has a greater impact on the yield. When it is 40 equivalents, the yield reaches 65%, and when it is more than 100 equivalents, it reaches more than 80%. Considering the cost problem, you can choose about 50 equivalents; similarly, The amount of heavy water has little effect on the deuterium rate. With the increase of the heavy water amount, the deuterium rate increases from 95% to 98.7%. Above 30 equivalents, the deuterium rate is greater than or equal to 98.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com