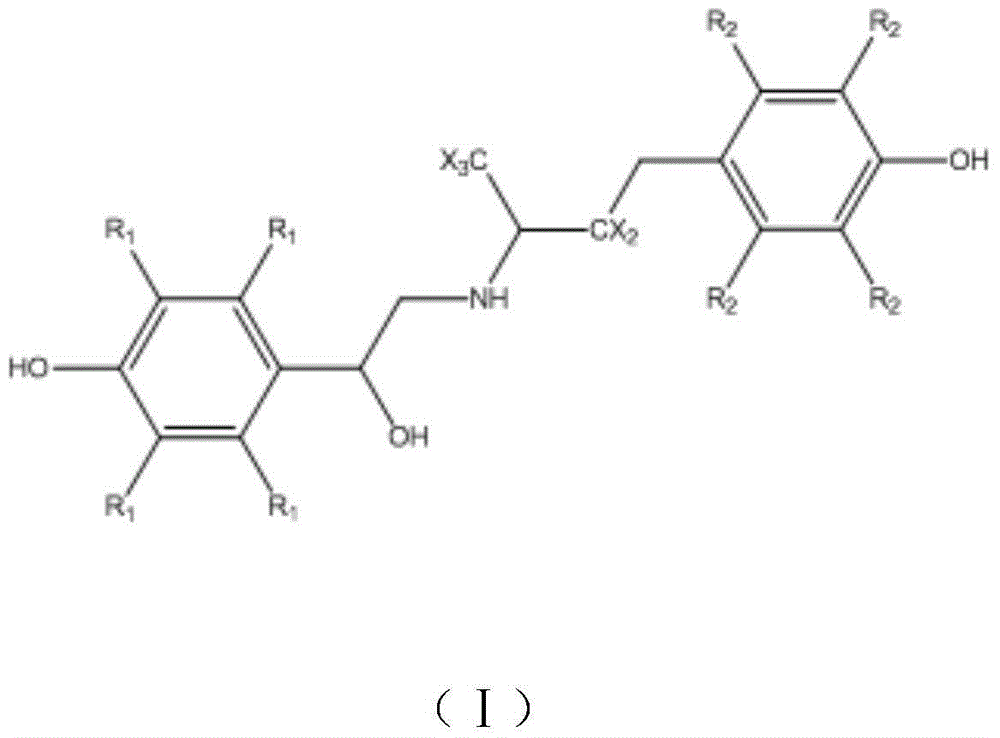
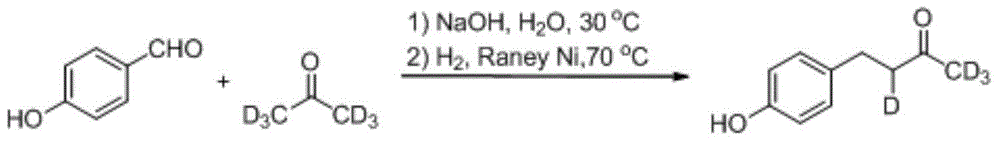Synthesis method for deuterium marked ractopamine
A technique for ractopamine and a synthetic method, which is applied in chemical instruments and methods, preparation of organic compounds, preparation of aminohydroxyl compounds, etc., can solve problems such as severe reaction conditions and cumbersome reaction routes, achieve simple synthesis steps, reduce production costs, The effect of temperature control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
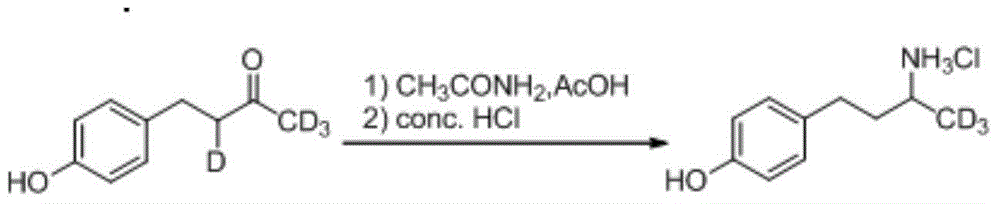
[0026] (1) The synthetic method of deuterium-labeled raspberry ketone
[0027]
[0028] In a 100mL three-necked flask equipped with a stirrer and a thermometer, add deuterium-labeled acetone (5.77g, 90mmol), sodium hydroxide (0.8g, 20mmol), and 3.5mL of distilled water. Formaldehyde solution (among them, p-hydroxybenzaldehyde (7.33g, 60mmol), sodium hydroxide (2.44g, 61mmol), distilled water 57.5mL), the temperature was controlled at 30°C, the addition was completed in 20min, and the stirring reaction was continued for 3h; Neutralize to pH=5~6, add activated carbon for decolorization, and filter. After the filtrate was cooled and crystallized, the filter cake was filtered and dried to obtain a deuterium-labeled unsaturated ketone (7.88 g, 79%).
[0029] Put deuterium-labeled unsaturated ketone (3.0g, 18mmol), absolute ethanol 174mL, and Raney Ni 1g into a stainless steel autoclave, and pass H 2 (1MPa). After stirring at 70-80° C. for 2 h, the reaction solution was filter...
Embodiment 2
[0045] (1) The synthetic method of phenyl ring deuterium label raspberry ketone
[0046]
[0047] In a 100mL three-necked flask equipped with a stirrer and a thermometer, add acetone (7.9mL, 108mmol), sodium hydroxide (0.8g, 20mmol), and 3.5mL of distilled water. After stirring evenly, slowly add deuterium-labeled p-hydroxybenzene Formaldehyde solution (deuterium-labeled p-hydroxybenzaldehyde (7.56g, 60mmol), sodium hydroxide (2.44g, 61mmol), distilled water 57.5mL), the temperature was controlled at 30°C, the addition was completed in 20min, and the stirring was continued for 3h. After the reaction, neutralize with mineral acid to pH=5-6, add active carbon for decolorization, and filter. After the filtrate was cooled and crystallized, the filter cake was filtered out and dried to obtain a deuterium-labeled unsaturated ketone (8.4 g, 83%).
[0048] Put deuterium-labeled unsaturated ketone (3.0g, 18mmol), absolute ethanol 174mL, and Raney Ni 1g into a stainless steel autocl...
Embodiment 3
[0054] (1) The synthetic method of deuterium-labeled raspberry ketone is the same as in Example 1.
[0055] (2) The synthesis method of deuterium-labeled 1-methyl-3-(4-hydroxyphenyl)-propylamine hydrochloride is the same as in Example 1.
[0056] (3) The synthetic method of deuterium-labeled acetophenone amines intermediate
[0057]
[0058] Add 1-methyl-3-(4-hydroxyphenyl)-propylamine hydrochloride (5.3g, 32mmol), deuterium-labeled ω-bromo-p-hydroxyacetophenone (5.4g, 25mmol) into a 250mL three-necked flask, Sodium carbonate (3.4g, 32mmol), ethyl acetate 30mL and saturated sodium carbonate aqueous solution 50mL, after mechanically stirring rapidly for 2.5h, add deuterium-labeled ω-bromo-p-hydroxyacetophenone (1.3g, 6mmol) in ethyl acetate solution 10mL , Continue to react at 60°C for 2h. Filter, transfer the filter cake to a 50mL flask, add 19mL of concentrated hydrochloric acid and 19mL of water, and reflux for 30min. After cooling, filter and wash the filter cake with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com