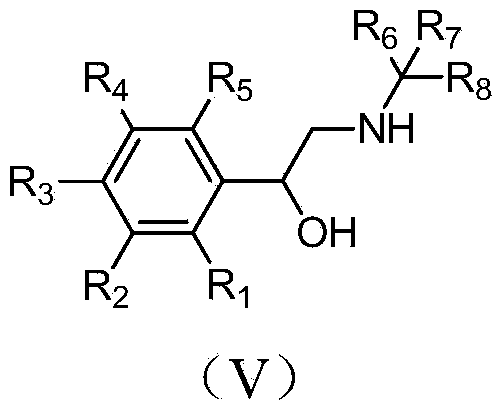
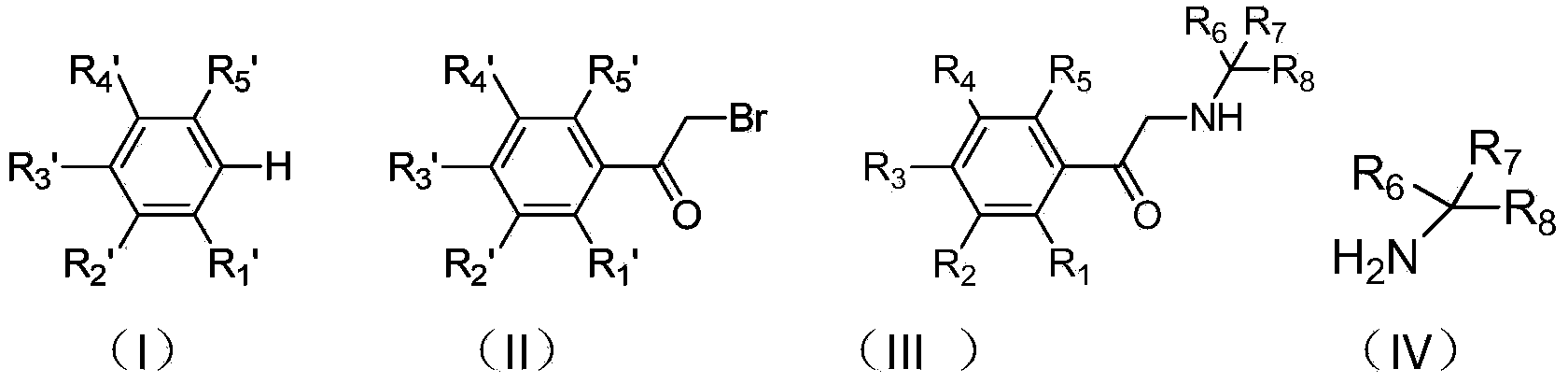
Synthetic method of deuterium-labeled phenylethanolamine beta receptor stimulants
A technology of phenylethanolamine and synthesis method, applied in chemical instruments and methods, formation/introduction of hydroxyl groups, preparation of organic compounds, etc., can solve problems such as difficulty in using phenylethanolamine compounds, cumbersome steps, long synthesis route, etc., and achieve synthesis The method is simple and efficient, and the effect of high deuterium substitution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: six deuterium labeled salbutamol
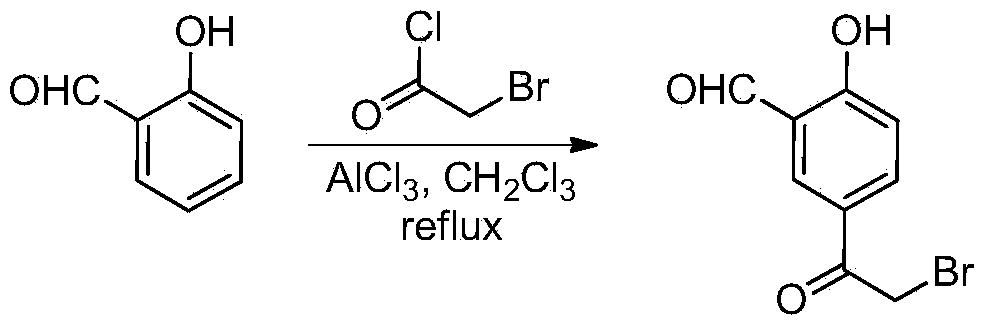
[0027] (1) The synthetic method of 5-(2-bromoacetyl)-2-hydroxybenzaldehyde
[0028]
[0029] Add anhydrous aluminum trichloride (33.3g, 250mmol) and 35mL of dichloromethane into a 100mL three-necked flask, add 7.5mL of a dichloromethane solution of bromoacetyl chloride (9.9g, 63mmol) dropwise under reflux, stir for 0.5h and slowly 7.5 mL of a solution of salicylaldehyde (6.1 g, 50 mmol) in dichloromethane was added dropwise. Refluxed for 16h, poured into ice water to quench, and separated. The aqueous phase was extracted three times with dichloromethane, and the combined organic phases were washed with water and saturated brine and dried over anhydrous sodium sulfate. After filtration, the solvent was spin-dried, and the solid was washed with dichloromethane and diethyl ether to obtain ω-bromo-acetophenone intermediate 5-(2-bromoacetyl)-2-hydroxybenzaldehyde (8.3 g, 70%). 1 H NMR (300MHz, CDCl 3 )δ10.36(1H,s),8.05(...
Embodiment 2
[0037] Embodiment 2: Nine deuterium-labeled salbutamol
[0038] (1) Synthesis of nonadeuterium-labeled 5-(2-tert-butylaminoacetyl)-2-hydroxybenzaldehyde hydrochloride from 5-(2-bromoacetyl)-2-hydroxybenzaldehyde
[0039]
[0040] Add 5-(2-bromoacetyl)-2-hydroxybenzaldehyde (10.8g, 44.4mmol) and 40mL isopropanol into a 100mL flask, stir and add tert-butylamine-d under nitrogen 9 (10.5g, 133mmol), refluxed for 2h. Add 11.1mL of 12mol / L hydrochloric acid and 9mL of isopropanol, and continue to reflux for 18h. Cool to room temperature and filter. The filter cake was stirred in 100 mL of isopropanol for 18 h and then filtered to obtain the nine-deuterium-labeled acetophenone amine intermediate 5-(2-tert-butylaminoacetyl)-2-hydroxybenzaldehyde hydrochloride (5.8 g, 47%) . 1 H NMR (300MHz, CDCl 3 )δ10.36(1H,s),8.05(1H,d,J=7.2Hz),7.58(1H,s),7.25(1H,d,J=7.2Hz),5.35(1H,br),3.65( 2H, s).
[0041] (2) Synthesis of nonadeuterium-labeled albuterol from nonadeuterium-labeled 5-(2-t...
Embodiment 3
[0045] Example 3: Hexadeuterium Labeled Clenbuterol
[0046] (1) The synthetic method of 5-(2-bromoacetyl)-2,5-dichloroaniline
[0047]
[0048] Add anhydrous aluminum trichloride (33.3g, 250mmol) and 35mL of dichloromethane into a 100mL three-necked flask, add 7.5mL of a dichloromethane solution of bromoacetyl chloride (9.9g, 63mmol) dropwise under reflux, stir for 0.5h and slowly 7.5 mL of a dichloromethane solution of 2,5-dichloroaniline (8.0 g, 50 mmol) was added dropwise. Refluxed for 16h, poured into ice water to quench, and separated. The aqueous phase was extracted three times with dichloromethane, and the combined organic phases were washed with water and saturated brine and dried over anhydrous sodium sulfate. After filtration, the solvent was spin-dried, and the solid was washed with dichloromethane and ether to obtain ω-bromo-acetophenone intermediate 5-(2-bromoacetyl)-2,5-dichloroaniline (9.1g, 64% ). 1 H NMR (300MHz, CDCl 3 )δ7.51 (2H, s), 6.30 (2H, br), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com