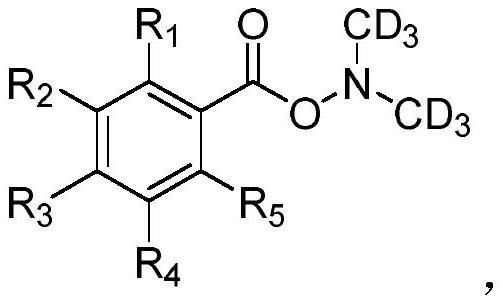
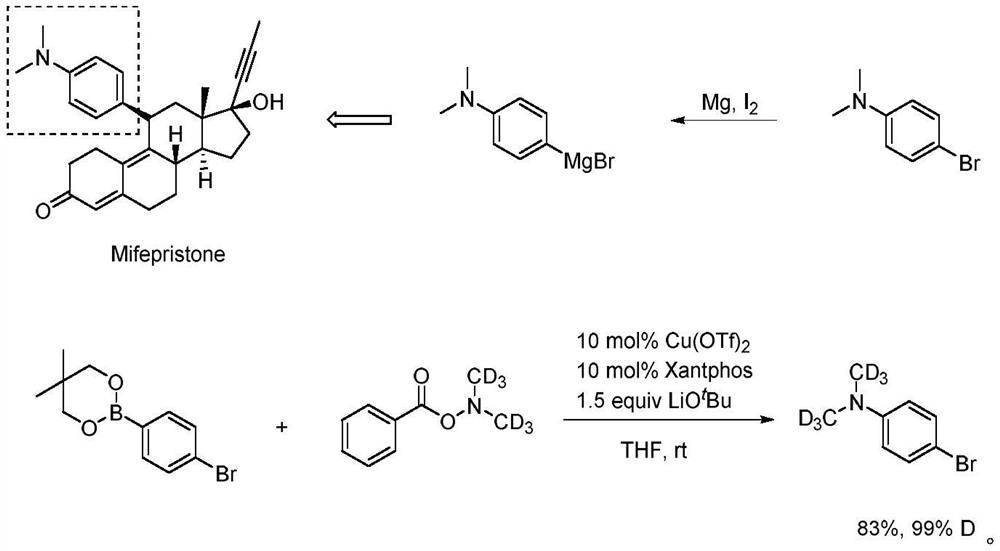
A kind of deuterated dimethylhydroxylamine benzoate compound and its preparation method and application
A technology for dimethylhydroxylamine benzoic acid and ester compounds, which is applied in the field of novel deuterated dimethylhydroxylamine benzoic acid ester compounds and their preparation, and achieves the effects of fast reaction rate, high yield and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] O-benzoyl-N,N-di(methyl-d 3 ) Preparation of hydroxylamine:
[0037] 100mL Schlenk tube, replaced with argon three times. Add 600mg (15mmol) of sodium hydride (NaH) and 10mL of anhydrous N,N-dimethylformamide under the protection of argon, and cool to 0°C. Then 600 mg (5 mmol) of tert-butyl carbamate was dissolved in 10 mL of anhydrous N,N-dimethylformamide, and slowly injected into the Schlenk tube. Finally, 2.2 g (15 mmol) of deuteroiodomethane was dissolved in 10 mL of anhydrous N,N-dimethylformamide, slowly injected into the Schlenk tube, and reacted at 0° C. for 3 hours. After the reaction was completed, the reaction solution was quenched with water (150 mL), followed by extraction with 50 mL of diethyl ether three times. The organic phase was collected and washed twice with 50 mL of water. Concentration of the organic phase afforded tert-butylbis(methyl-d 3 ) carbamate.
[0038] The resulting tert-butylbis(methyl-d 3 ) carbamate, cooled to 0°C, and 10 mL of...
Embodiment 2
[0042] O-(4-fluorobenzoyl)-N,N-di(methyl-d 3 ) Preparation of hydroxylamine:
[0043] 100mL Schlenk tube, replaced with argon three times. Add 600mg (15mmol) of sodium hydride (NaH) and 10mL of anhydrous N,N-dimethylformamide under the protection of argon, and cool to 0°C. Then 600 mg (5 mmol) of tert-butyl carbamate was dissolved in 10 mL of anhydrous N,N-dimethylformamide, and slowly injected into the Schlenk tube. Finally, 2.2 g (15 mmol) of deuteroiodomethane was dissolved in 10 mL of anhydrous N,N-dimethylformamide, slowly injected into the Schlenk tube, and reacted at 0° C. for 3 hours. After the reaction was completed, the reaction solution was quenched with water (150 mL), followed by extraction with 50 mL of diethyl ether three times. The organic phase was collected and washed twice with 50 mL of water. Concentration of the organic phase afforded tert-butylbis(methyl-d 3 ) carbamate.
[0044] The resulting tert-butylbis(methyl-d 3 ) carbamate, cooled to 0°C, an...
Embodiment 3
[0048] O-(3,4-dichlorobenzoyl)-N,N-di(methyl-d 3 ) Preparation of hydroxylamine:
[0049] 100mL Schlenk tube, replaced with argon three times. Add 600mg (15mmol) of sodium hydride (NaH) and 10mL of anhydrous N,N-dimethylformamide under the protection of argon, and cool to 0°C. Then 600 mg (5 mmol) of tert-butyl carbamate was dissolved in 10 mL of anhydrous N,N-dimethylformamide, and slowly injected into the Schlenk tube. Finally, 2.2 g (15 mmol) of deuteroiodomethane was dissolved in 10 mL of anhydrous N,N-dimethylformamide, slowly injected into the Schlenk tube, and reacted at 0° C. for 3 hours. After the reaction was completed, the reaction solution was quenched with water (150 mL), followed by extraction with 50 mL of diethyl ether three times. The organic phase was collected and washed twice with 50 mL of water. Concentration of the organic phase afforded tert-butylbis(methyl-d 3 ) carbamate.
[0050] The resulting tert-butylbis(methyl-d 3 ) carbamate, cooled to 0°C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com