Synthesis method of aryl deuterated difluoromethyl compound
A synthesis method and difluoromethyl technology, which are applied in the field of synthesis of aryl deuterated difluoromethyl compounds, can solve the problems of few types of aryl deuterated difluoromethyl compounds, poor substrate adaptability, etc., and achieve applicable Effects with a wide range, easy operation, and simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
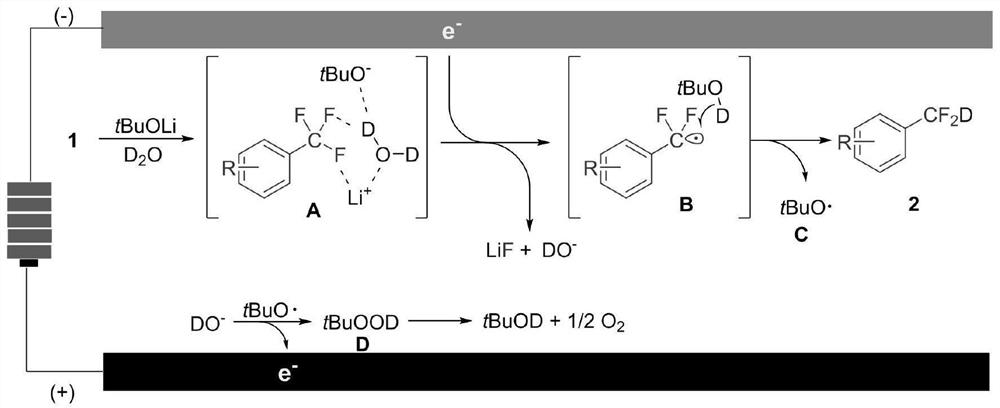
[0037] The invention provides a kind of synthetic method of aryl deuterated difluoromethyl compound, comprising the following steps:
[0038] An aryl trifluoromethyl compound, an electrolyte, an activator, heavy water and a solvent are mixed for an electrolysis reaction to obtain an aryl deuterated difluoromethyl compound.
[0039] In the present invention, the general structural formula of the aryl trifluoromethyl compound is shown in formula I:
[0040] Ar-CF 3 Formula I;
[0041] In formula I: Ar represents phenyl, substituted phenyl, heteroaryl or heteroaryl; the substituents in the substituted phenyl include alkoxy, aryloxy, halogen, borate ester, silyl, Thioether group, NH 2 -, amino, acylamino, furyl, phenyl, substituted phenyl or amido; the alkyl in the alkoxy is preferably an alkanyl or cycloalkyl, and the number of carbon atoms in the alkoxy is preferably is 1 to 12, more preferably 3 to 10; the aryloxy group is preferably phenoxy, substituted phenoxy, naphthylo...
Embodiment 1
[0070] The raw material that adopts is compound 1a, and product is compound 2a, and reaction formula is as follows:
[0071]
[0072] Add compound 1a (0.2mmol), tetrabutylammonium bromide (0.2mmol), lithium tert-butoxide (0.2mmol) in two heart-shaped flasks, place between two pieces of graphite felt (1 × 1 × 0.5cm) A polytetrafluoroethylene film to ensure that the distance between the poles is not less than 0.2mm. Put two pieces of graphite felt into two heart-shaped bottles, connect a platinum wire to one piece of graphite felt, connect the positive pole of the power supply as an anode, and connect a piece of graphite felt to the anode. Insert a silver wire to connect the negative pole of the power supply as the cathode. After replacing the atmosphere in the reaction bottle with argon, add 5mL of N,N-dimethylformamide and heavy water (10mmol), and apply a voltage of 3.6V on the cathode and anode, that is, the voltage between the platinum wire and the silver wire is +3.6V, ...
Embodiment 2~37
[0077] Other conditions are the same as in Example 1, only the raw material compound 1a is replaced, the reaction voltage is adjusted, the reaction is monitored by gas chromatography to the end, and the reaction time is recorded; the raw material used in each embodiment is the -CDF in the product 2 Replace with -CF 3 After the compound, the specific structure is no longer listed one by one; in addition, the amount of 2n, 2ai, 2aj, 2an, 2at group reaction substrate is 0.1mmol, the amount of tetrabutylammonium bromide, lithium tert-butoxide and heavy water It is also halved on the basis of Example 1; the reaction temperature of Group 2an is 15° C., and the conditions not listed are all consistent with Example 1.
[0078]Product structure, reaction conditions and productive rate, deuterium substitution rate in the embodiment are shown in Table 1:
[0079] The product structure, reaction conditions, productive rate and deuterated rate of table 1 embodiment 2~37
[0080]
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com