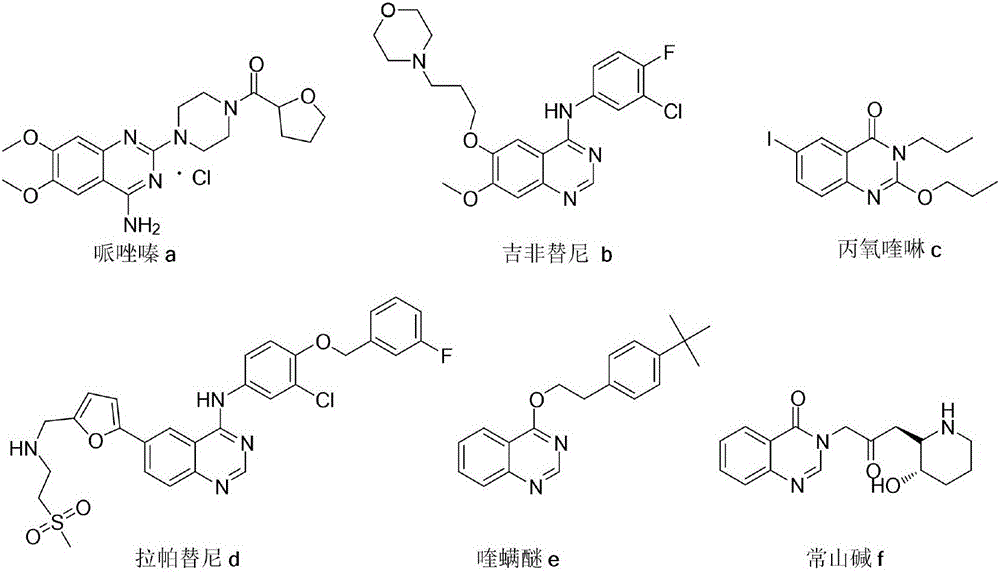
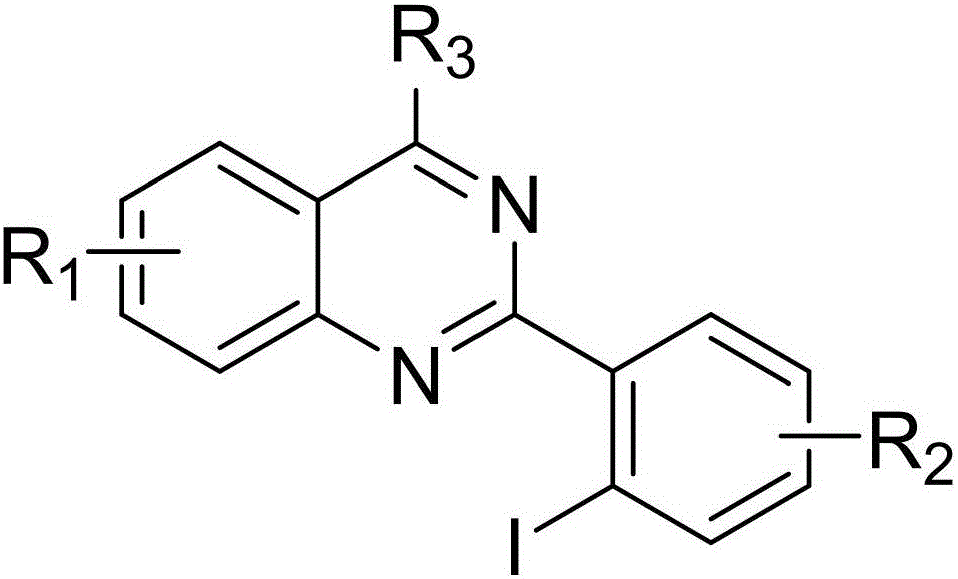
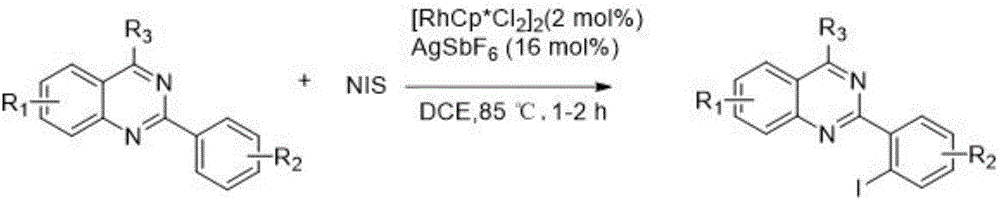
2-(2-iodoaryl)quinazoline compound and preparation method thereof
A quinazoline and iodo aryl technology is applied in the field of 2-quinazoline compounds and their preparation, which can solve the problems of miscellaneous products and difficult separation, and achieves convenient operation, easy column chromatography separation, and good application. effect of value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0048]4-phenyl-2-p-tolylquinazoline (0.3mmol, 1.0eq), iodosuccinimide (NIS) (0.45mmol, 1.5eq), dichloro(pentamethylcyclopentadiene Base) rhodium (III) dimer (0.006mmol, 0.02equiv) and silver hexafluoroantimonate (0.024mmol, 0.08equiv) were added to the reaction test tube, and then the organic solvent 1,2-dichloroethane (2mL) was added React at 85°C for 1 hour. It was detected by TLC that the reaction was complete, cooled to room temperature, extracted with ethyl acetate and water, and the solvent was spin-dried, and the crude product was separated and purified by flash column chromatography with eluent (petroleum ether / ethyl acetate=30 / 1) to obtain pure Final product 2-(2-iodo-p-tolyl)-4-phenylquinazoline (2a).
[0049]
[0050] Yield: 87%; mp: 143-146°C;
[0051] 1 H NMR (400MHz, CDCl 3 ):δ8.20-8.18(m,2H),7.95-7.91(m,3H),7.90-7.88(m,1H),7.78(s,1H),7.65-7.57(m,4H),7.29(d ,J=8.00Hz,1H),2.37(s,3H);
[0052] 13 C NMR (100MHz, CDCl 3 ): δ168.0, 163.1, 151.5, 140.9, 140....
example 2
[0055] 2,4-diphenylquinazoline (0.3mmol, 1.0eq), NIS (0.45mmol, 1.5eq), dichloro(pentamethylcyclopentadienyl) rhodium (III) dimer (0.006 mmol, 0.02 equiv) and silver hexafluoroantimonate (0.024 mmol, 0.08 equiv) were added to the reaction test tube, and then the organic solvent 1,2-dichloroethane (2 mL) was added to react at 85°C for 1.5 hours. It was detected by TLC that the reaction was complete, cooled to room temperature, extracted with ethyl acetate and water, and the solvent was spin-dried, and the crude product was separated and purified by flash column chromatography with eluent (petroleum ether / ethyl acetate=30 / 1) to obtain pure The final product is 2-(2-iodophenyl)-4-phenylquinazoline (2b).
[0056]
[0057] Yield: 81%; mp: 123-124℃;
[0058] 1 H NMR (400MHz, CDCl 3 ): δ8.20(d, J=8.4Hz, 2H), 8.03(d, J=8.0Hz, 1H), 7.97-7.93(m, 3H), 7.87(dd, J=1.6, 7.6Hz, 1H) ,7.67-7.63(m,1H),7.60-7.57(m,3H),7.51-7.47(m,1H),7.15-7.11(m,1H);
[0059] 13 C NMR (100MHz, CDCl 3 )...
example 3
[0062] Combine 2-(4-methoxyphenyl)-4-phenylquinazoline (0.3mmol, 1.0eq), NIS (0.45mmol, 1.5eq), dichloro(pentamethylcyclopentadienyl) Rhodium (III) dimer (0.006mmol, 0.02equiv) and silver hexafluoroantimonate (0.024mmol, 0.08equiv) were added to the reaction test tube, and then the organic solvent 1,2-dichloroethane (2mL) was added at 85°C Reaction 2.0 hours. It was detected by TLC that the reaction was complete, cooled to room temperature, extracted with ethyl acetate and water, and the solvent was spin-dried, and the crude product was separated and purified by flash column chromatography with eluent (petroleum ether / ethyl acetate=30 / 1) to obtain pure Final product 2-(2-iodo-4-methoxyphenyl)-4-phenylquinazoline (2c).
[0063]
[0064] Yield: 87%; mp: 185-186°C;
[0065] 1 H NMR (400MHz, CDCl 3 ):δ8.19-8.17(m,2H),7.94-7.91(m,3H),7.89-7.87(m,2H),7.58-7.56(m,4H),7.03(dd,J=2.4,8.4Hz ,1H),3.84(s,3H);
[0066] 13 C NMR (100MHz, CDCl 3 ): δ168.0, 162.7, 160.3, 151.5, 137....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com