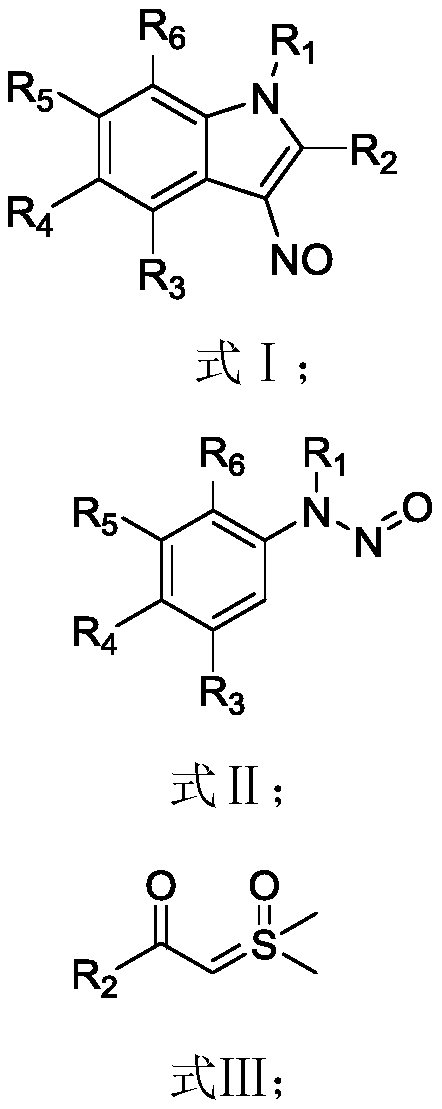
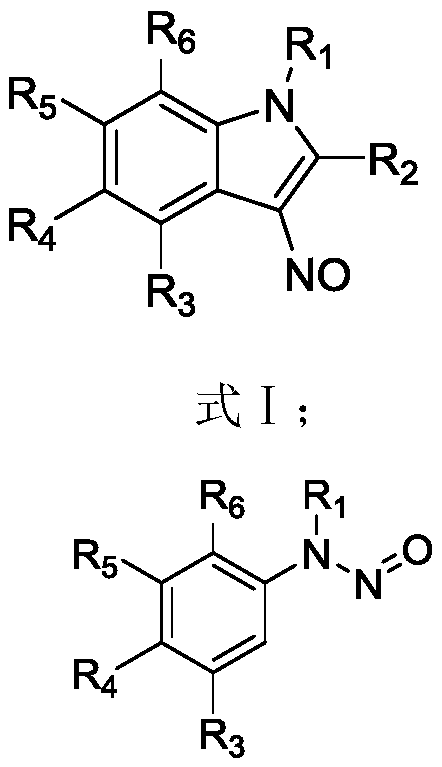
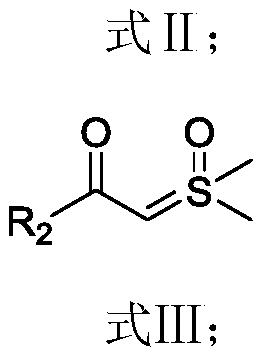
Preparation method of 3-nitroso substituted indole derivative
A technology of indole derivatives and nitroso groups, which is applied in the field of preparation of indole derivatives, can solve problems such as cumbersome preparation process, and achieve the effects of simple preparation process, easy control and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] The preparation method of the 3-nitroso substituted indole derivatives of the present embodiment comprises the following steps: 0.1 mmol of N-methyl-N-nitrosoaniline, 0.15 mmol of benzoylsulfide ylide, dichloro(penta Add 0.005 mmol of methylcyclopentadienyl) rhodium (III) and 0.02 mmol of silver carbonate to a Shrek tube, dissolve in 1.0 mL of hexafluoroisopropanol solvent, react at 40°C for 9 hours, and then add to the reaction solution 0.25mmol of trifluoroacetic acid was added to the mixture, and the reaction was continued for 4h. The reaction solution was filtered through diatomaceous earth, the filtrate was concentrated, and purified by column chromatography to obtain 20.3 mg of the target product. The yield of the target product was 86%.
[0061] The 3-nitroso-substituted indole derivative obtained by the preparation method of this embodiment is 1-methyl-3-methylene-2-phenyl-1H-indole, and its structural formula is:
[0062]
Embodiment 2
[0064] The preparation process of the 3-nitroso-substituted indole derivatives of this embodiment refers to the process in Example 1, the only difference is that the benzoylthio ylide is replaced by 4-methoxybenzoylthio ylide, and the rest are the same. In this example, the reaction solution after the reaction was filtered through diatomaceous earth, the filtrate was concentrated, and purified by column chromatography to obtain 20 mg of the target product. The yield of the target product was 75%.
[0065] The 3-nitroso-substituted indole derivative obtained by the preparation method of this embodiment is 2-(4-methoxyphenyl)-1-methyl-3-nitroso-1H-indole, which The structural formula is:
[0066]
Embodiment 3
[0068] The preparation process of the 3-nitroso-substituted indole derivatives of this embodiment refers to the process in Example 1, the only difference is that the benzoyl sulfide ylide is replaced by 4-methylbenzoyl thio ylide, and the rest are same. In this example, the reaction solution after the reaction was filtered through diatomaceous earth, the filtrate was concentrated, and purified by column chromatography to obtain 20 mg of the target product. The yield of the target product was 80%.
[0069] The 3-nitroso-substituted indole derivative obtained by the preparation method of this embodiment is 1-methyl-3-nitroso-2-(p-tolyl)-1H-indole, and its structural formula is:
[0070]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com