Compound based on chiral binaphthol-terpyridyl platinum and preparation method of compound
A technology of binaphthol and terpyridine, applied in the field of chiral fluorescent materials, can solve problems such as limited application, and achieve the effects of low synthesis cost, simple synthesis route and potential application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Synthesis of compound BTPt-Cl:
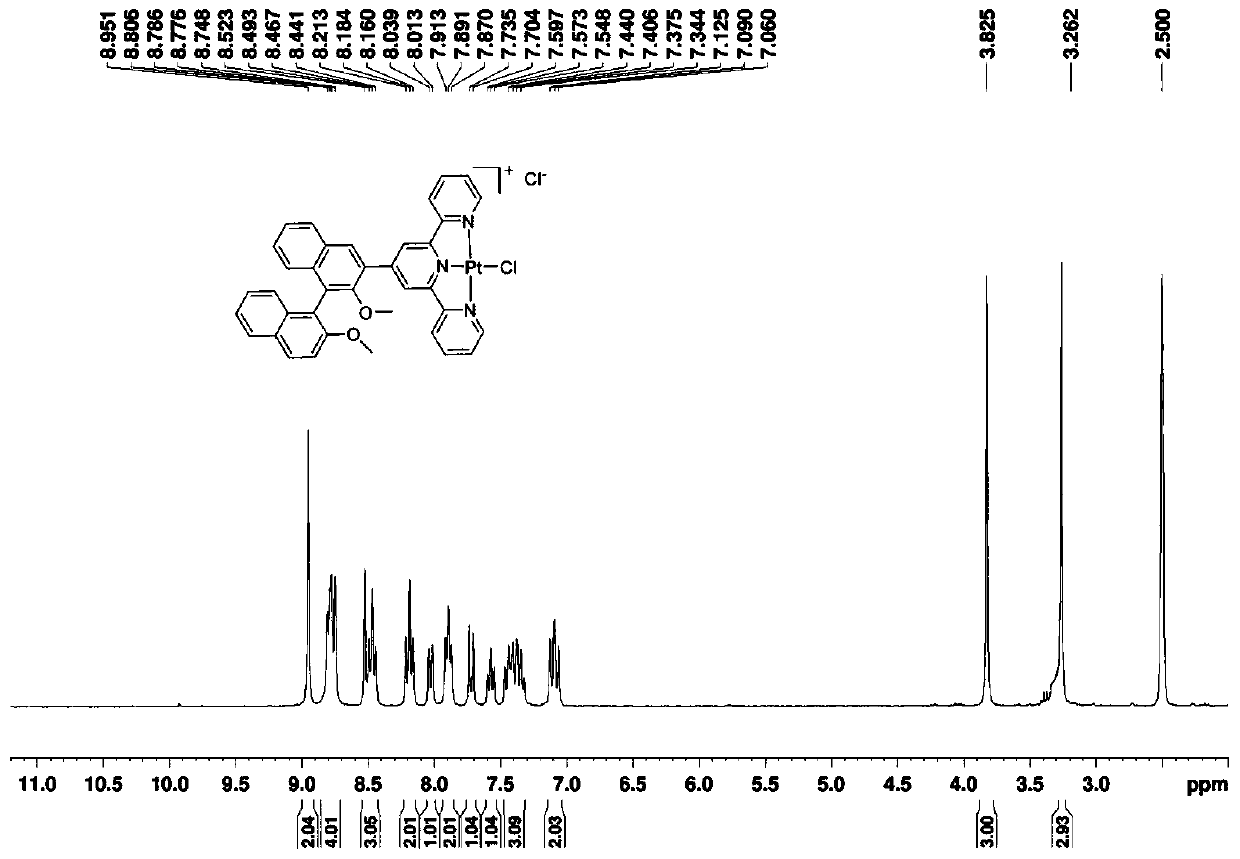
[0040] Potassium chloroplatinite (100mg, 0.242mmol) was added to the reactor, dissolved in deionized water (12mL), and then compound BT (153mg, 0.242mmol) dissolved in acetonitrile (36mL) was added, heated to reflux for 24 hours to react A large amount of red suspended matter was generated in the container, and then the heating was stopped. After the reaction system was cooled to room temperature, the crude product was obtained by suction filtration, and then the compound BTPt-Cl was obtained by column chromatography with a developer of dichloromethane / methanol at a volume ratio of 15:1. (82 mg, 61% yield).
[0041] like figure 1 as shown, 1 H NMR (300MHz, DMSO-d 6 )δ / ppm=8.95(s,2H),8.78(dd,J=10.2,7.1Hz,4H),8.48(dd,J=16.1,8.4Hz,3H),8.19(t,J=8.0Hz,2H ), 8.03(d, J=7.8Hz, 1H), 7.89(t, J=6.5Hz, 2H), 7.72(d, J=9.2Hz, 1H), 7.57(t, J=7.4Hz, 1H), 7.39(dt,J=16.0,7.4Hz,3H),7.18–6.99(m,2H),3.83(s,3H),3.26(s,3H).
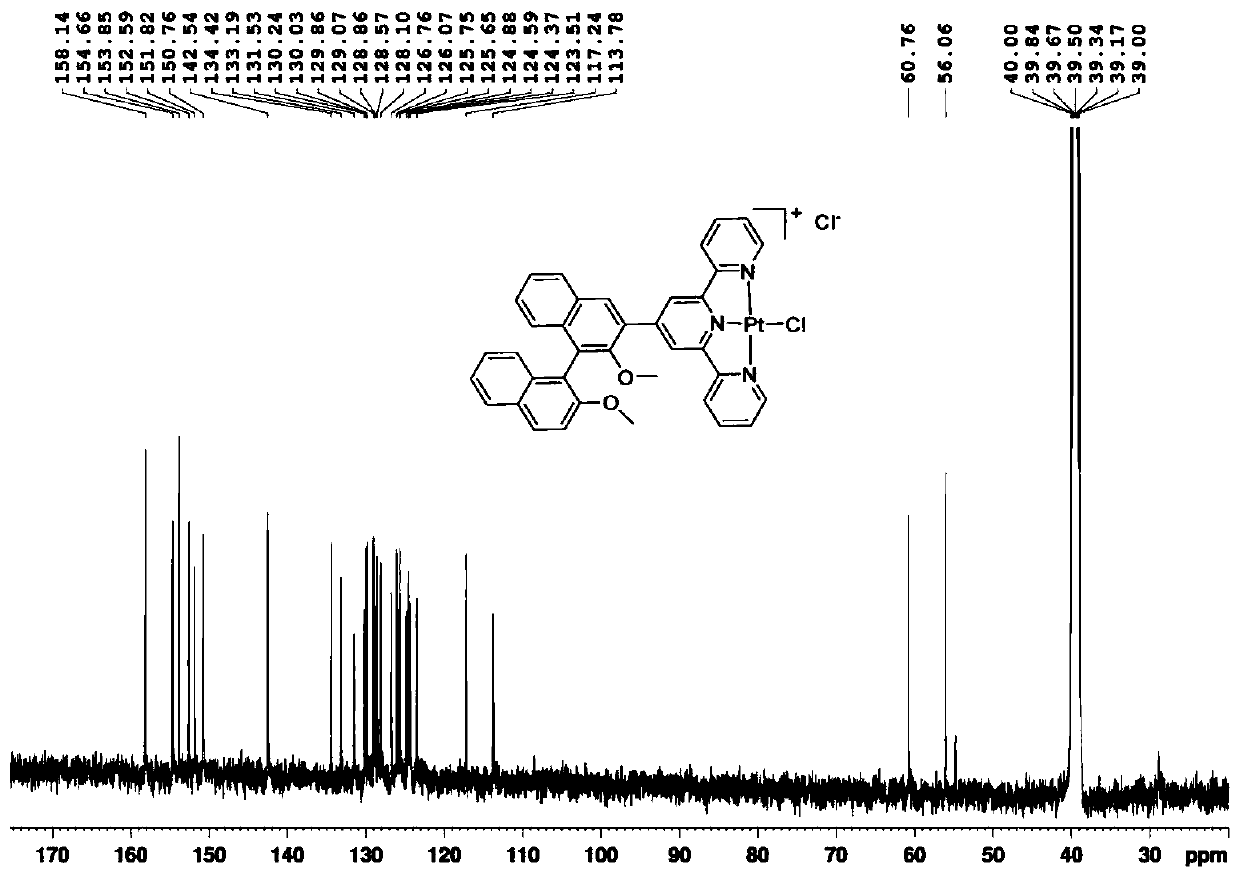
[0042] like figure 2 as sh...
Embodiment 2
[0048] Synthesis of compound BTPt-Cl:
[0049] Potassium chloroplatinite (301mg, 0.726mmol) was added to the reactor, dissolved in deionized water (18mL), and then compound BT (153mg, 0.242mmol) dissolved in acetonitrile (36mL) was added, heated to reflux for 48 hours to react A large amount of red suspended matter was generated in the container, and then the heating was stopped. After the reaction system was cooled to room temperature, the crude product was obtained by suction filtration, and then the compound BTPt-Cl was obtained by column chromatography with a developer of dichloromethane / methanol at a volume ratio of 15:1. .
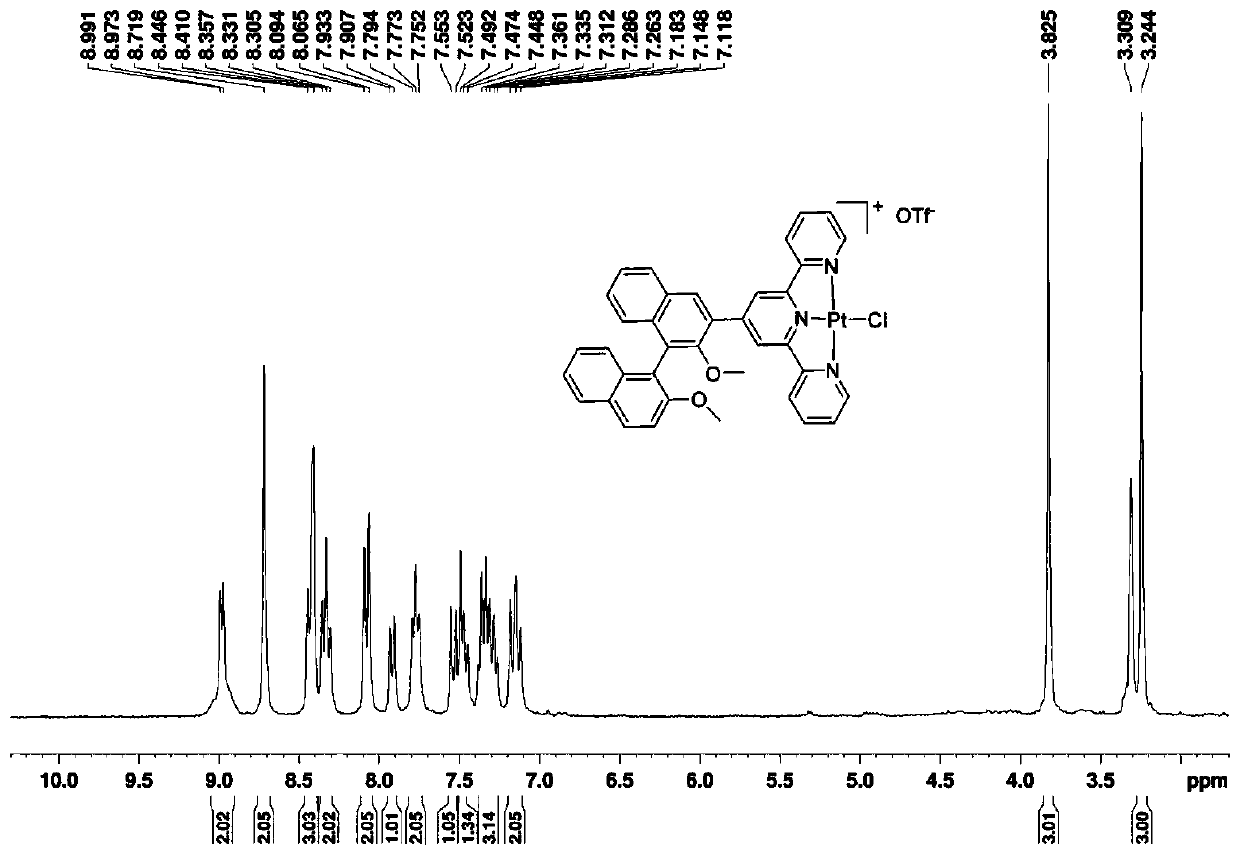
[0050] Synthesis of compound BTPt-OTf:
[0051] Compound BTPt-Cl (80mg, 0.1mmol) and silver trifluoromethanesulfonate (26mg, 0.1mmol) were added in the reactor, heated to reflux in 5mL of methanol for 2 hours, the system gradually changed from orange-red to gray-green at the beginning, After stopping the reaction. After the system was cooled to ro...
Embodiment 3
[0053] Synthesis of compound BTPt-Cl:
[0054] Potassium chloroplatinite (100mg, 0.242mmol) was added to the reactor, dissolved in deionized water (12mL), then compound BT (153mg, 0.242mmol) dissolved in acetonitrile (60mL) was added, heated to reflux for 18 hours to react A large amount of red suspended matter was generated in the container, and then the heating was stopped. After the reaction system was cooled to room temperature, the crude product was obtained by suction filtration, and then the compound BTPt-Cl was obtained by column chromatography with a developer of dichloromethane / methanol at a volume ratio of 15:1. .
[0055] Synthesis of compound BTPt-OTf:
[0056] Compound BTPt-Cl (80mg, 0.1mmol) and silver trifluoromethanesulfonate (77mg, 0.3mmol) were added to the reactor, heated to reflux in 5mL of methanol for 1 hour, the system gradually changed from orange-red to gray-green at the beginning, After stopping the reaction. After the system was cooled to room te...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com