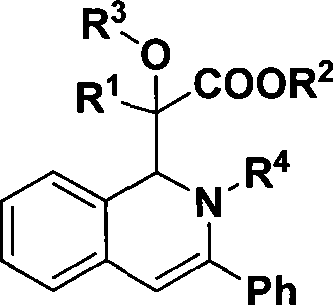
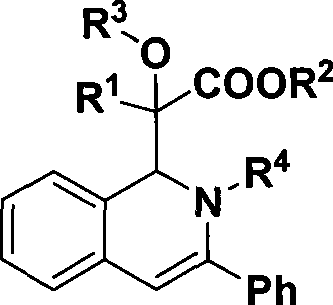
1,2-dihydroisoquinoline derivative and preparation thereof
A technology of dihydroisoquinoline and derivatives, applied in organic chemistry and other fields, can solve the problems of low yield and long steps, and achieve the effects of convenient operation, mild reaction conditions and easy preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Preparation of 2-(1,2-dihydro-2,3-diphenylisoquinolin-1-yl)-2-hydroxy-2-phenylacetic acid methyl ester
[0029] The preparation of mixed solution is: take by weighing 2-phenylethynyl-N-phenylbenzaldimine (87.1mg, 0.31mmol), measure water (6.3 μ l, 0.35mmol) with microsampler, then weigh rhodium acetate ( 1.50mg, 0.0035mmol), silver trifluoromethanesulfonate (4.4mg, 0.017mmol), put them into a 25ml two-necked bottle, and add 8ml of anhydrous dichloromethane.
[0030] Preparation of diazo solution: Weigh ethyl phenyldiazoacetate (61.6 mg, 0.35 mmol) and add 4 ml of anhydrous dichloromethane.
[0031] Draw up the diazo solution with a 5ml syringe, inject it into the mixed solution with the control of a peristaltic pump for 1 hour, remove dichloromethane by rotary evaporation at 40°C, and then pass column chromatography (eluent is petroleum ether:ethyl acetate=50:1 volume than) isolate the target product pure product (a mixture of diastereoisomers, column chromatography ca...
Embodiment 2
[0036] Preparation of methyl 2-(1,2-dihydro-2,3-diphenylisoquinolin-1-yl)-2-hydroxy-2-(4-methoxyphenyl)acetate
[0037] The preparation of the mixed solution was the same as in Example 1.
[0038] Preparation of diazo solution: Weigh 4-methoxyphenyl ethyl diazoacetate (72.2 mg, 0.35 mmol) and add 4 ml of anhydrous dichloromethane.
[0039] Draw up the diazo solution with a 5ml syringe, inject it into the mixed solution for 1 hour under the control of a peristaltic pump, remove dichloromethane by rotary evaporation at 40°C, and then pass column chromatography (eluent: petroleum ether: ethyl acetate = 30:1 volume ratio) to isolate the target product pure product (a mixture of diastereomers, column chromatography can not be separated) as shown in the structure of 4b;
[0040]
[0041] 0.4H), 3.83(s, 1.2H), 3.77(s, 1.8H), 3.53(s, 1.2H), 3.49(s, 0.6H)
[0042] Yield: 90%, d.r. value: 56:44
Embodiment 3
[0044] Preparation of methyl 2-(1,2-dihydro-2,3-diphenylisoquinolin-1-yl)-2-hydroxy-2-(4-methylphenyl)acetate
[0045] The preparation of the mixed solution was the same as in Example 1.
[0046] Preparation of diazo solution: Weigh 4-methylphenyldiazoacetate ethyl ester (66.6mg, 0.35mmol) and add 4ml of anhydrous dichloromethane. Draw up the diazo solution with a 5ml syringe, inject it into the mixed solution for 1 hour under the control of a peristaltic pump, remove the dichloromethane by rotary evaporation at 40°C, and then pass column chromatography (eluent: petroleum ether: ethyl acetate = 40:1 volume ratio) to isolate the target product pure product (a mixture of diastereoisomers, which cannot be separated by column chromatography) of the structure shown in 4c;
[0047]
[0048] 0.4H), 3.94(s, 1.8H), 3.85(s, 0.4H), 3.53(s, 1.2H), 3.47(s, 0.6H), 2.38(s, 1.2H), 2.32(s, 1.8H) )
[0049] Yield: 84%, d.r. value: 58:42
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com