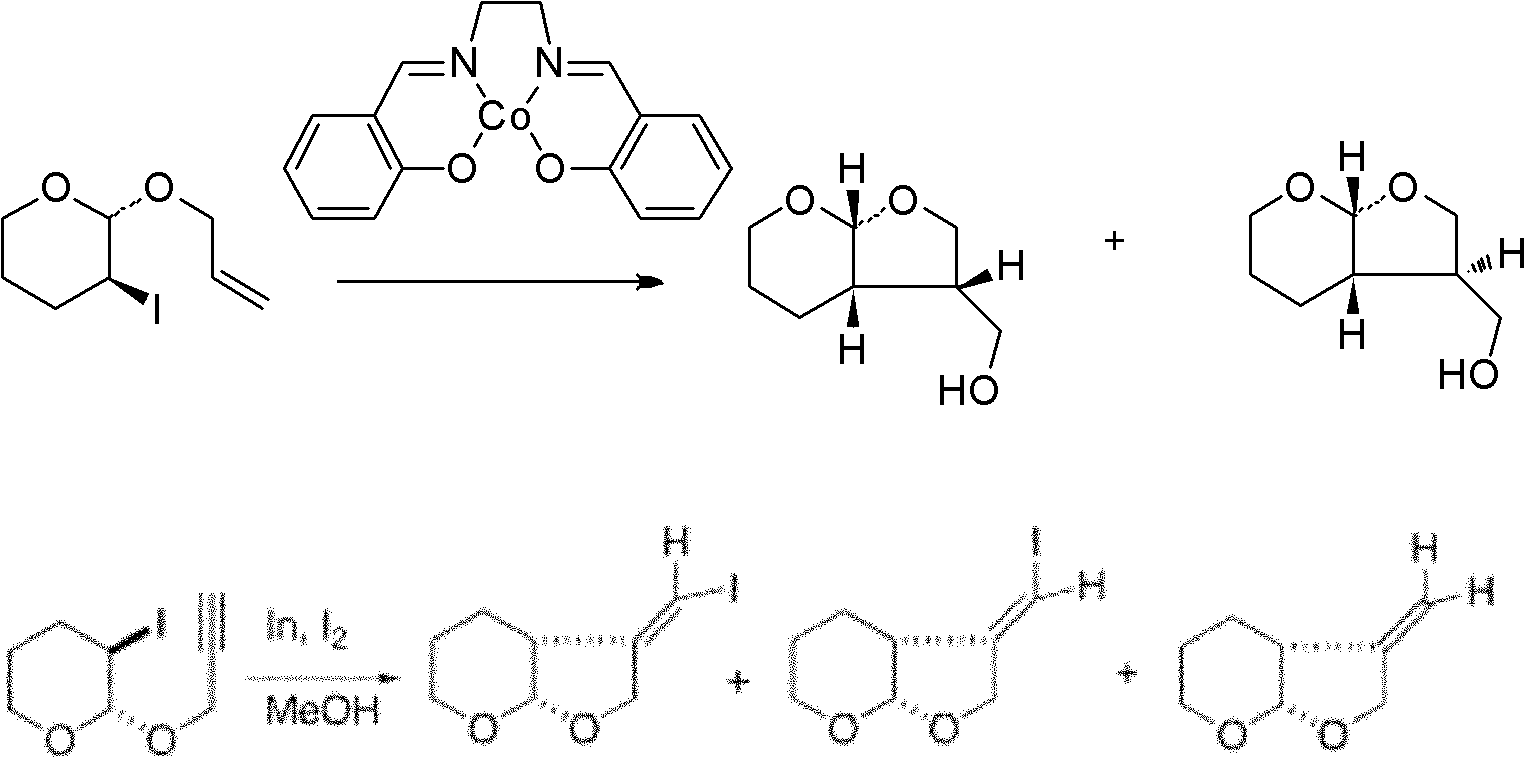
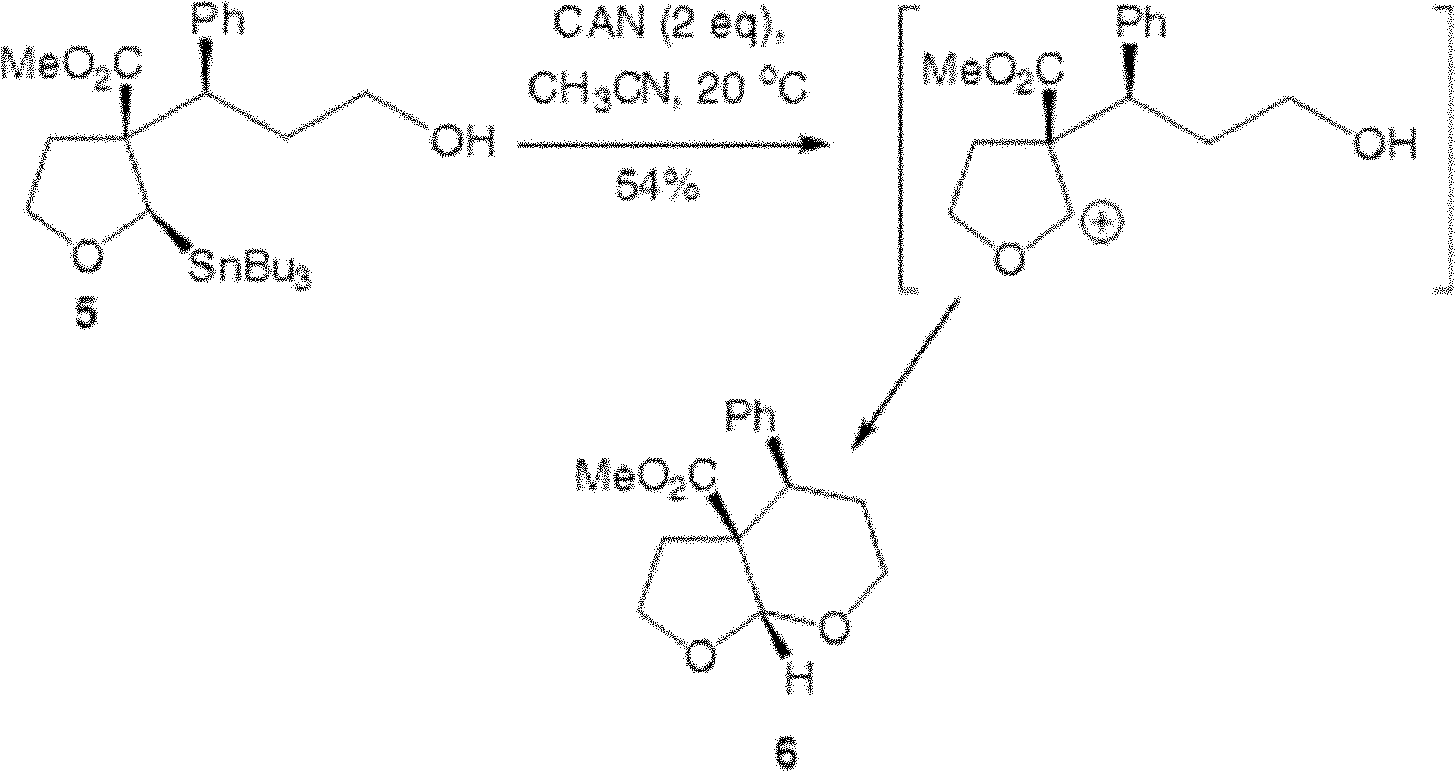
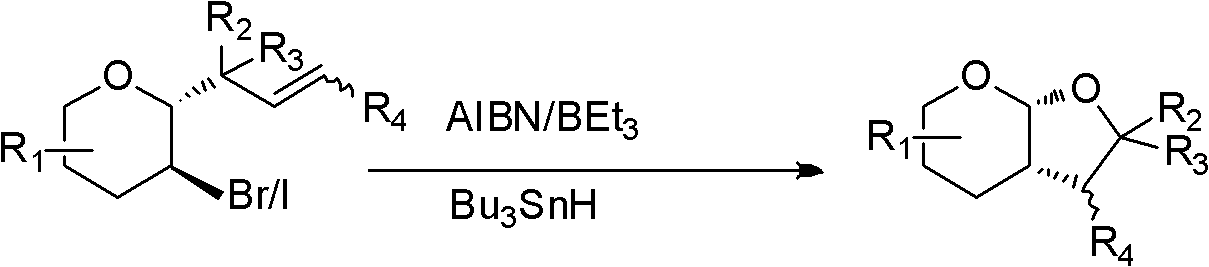Method for stereoselective preparation of derivatives of pyrane
A technology of pyran derivatives and stereoselectivity, which is applied in the preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc., can solve the problems of environmental pollution, rare reagents, and high cost, and achieve low environmental pollution and high selectivity. High and low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1: Under Ar protection, 1-ethylthio-2-C-acetaldehyde-α-D-glucopyranose derivatives 2 (52 mg, 0.1 mmol) and 2,3 were added to a 5 mL round bottom flask -O-isopropylidene-α-L-rhamnopyranoside (26.2mg, 0.12mmol), then add dry dichloromethane (2mL) to dissolve, then add to activate Molecular sieves (50 mg), stirred at room temperature for 30 minutes, the reaction temperature dropped to -30 ° C, added NIS (33.8 mg, 0.15 mmol), and then added trimethylsilyl trifluoromethanesulfonate (9.0 μ L, 0.05 mmol) Slowly heat up the reaction, and TLC detects that the reaction is complete. Add an appropriate amount of sodium thiosulfate to quench the reaction, then add dichloromethane (5mL) to dilute the reaction system, filter, the filtrate is washed with water (10mL×2), saturated brine (10mL×2), and then washed with anhydrous sodium sulfate dry. After filtration, the filtrate was concentrated and purified by silica gel column chromatography (eluent: petroleum ether / ethyl ace...
Embodiment 2
[0058] Example 2: Under Ar protection, 1-ethylthio-2-C-acetaldehyde-α-D-glucopyranose derivatives 2 (52 mg, 0.1 mmol) and 2,3 were added to a 5 mL round bottom flask -O-isopropylidene-α-L-rhamnopyranoside (26.2mg, 0.12mmol), then add dry dichloromethane (2mL) to dissolve, then add to activate Molecular sieves (50mg), stirred at room temperature for 30 minutes, the reaction temperature dropped to -30°C, NIS (33.8mg, 0.15mmol) was added, and silver trifluoromethanesulfonate (12.8mg, 0.05mmol) was added, and then the temperature was slowly raised to react. TLC detection reaction was completed. Add an appropriate amount of sodium thiosulfate to quench the reaction, then add dichloromethane (5mL) to dilute the reaction system, filter, the filtrate is washed with water (10mL×2), saturated brine (10mL×2), and then washed with anhydrous sodium sulfate dry. After filtration, the filtrate was concentrated and purified by silica gel column chromatography (eluent: petroleum ether / ethyl...
Embodiment 3
[0059] Example 3: Under Ar protection, 1-ethylthio-2-C-acetaldehyde-α-D-glucopyranose derivatives 2 (52 mg, 0.1 mmol) and 2,3 were added to a 5 mL round bottom flask -O-isopropylidene-α-L-rhamnopyranoside (26.2mg, 0.12mmol), then add dry dichloromethane (2mL) to dissolve, then add to activate Molecular sieves (50mg), stirred at room temperature for 30 minutes, the reaction temperature dropped to -30°C, NIS (33.8mg, 0.15mmol) was added, and trifluoromethanesulfonic acid (2.2μL, 0.025mmol) was added, and then the temperature was slowly raised to react, TLC Detection reaction to the end. Add an appropriate amount of sodium thiosulfate to quench the reaction, then add dichloromethane (5mL) to dilute the reaction system, filter, the filtrate is washed with water (10mL×2), saturated brine (10mL×2), and then washed with anhydrous sodium sulfate dry. After filtration, the filtrate was concentrated and purified by silica gel column chromatography (eluent: petroleum ether / ethyl aceta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com