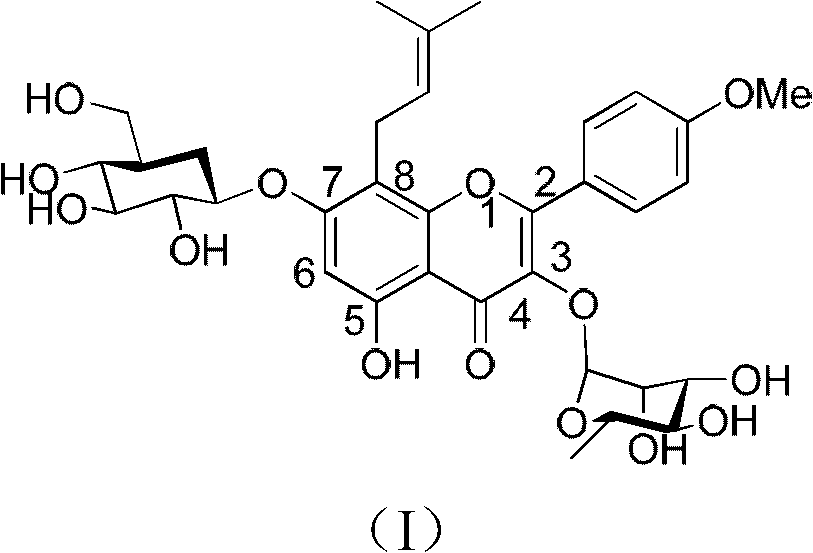
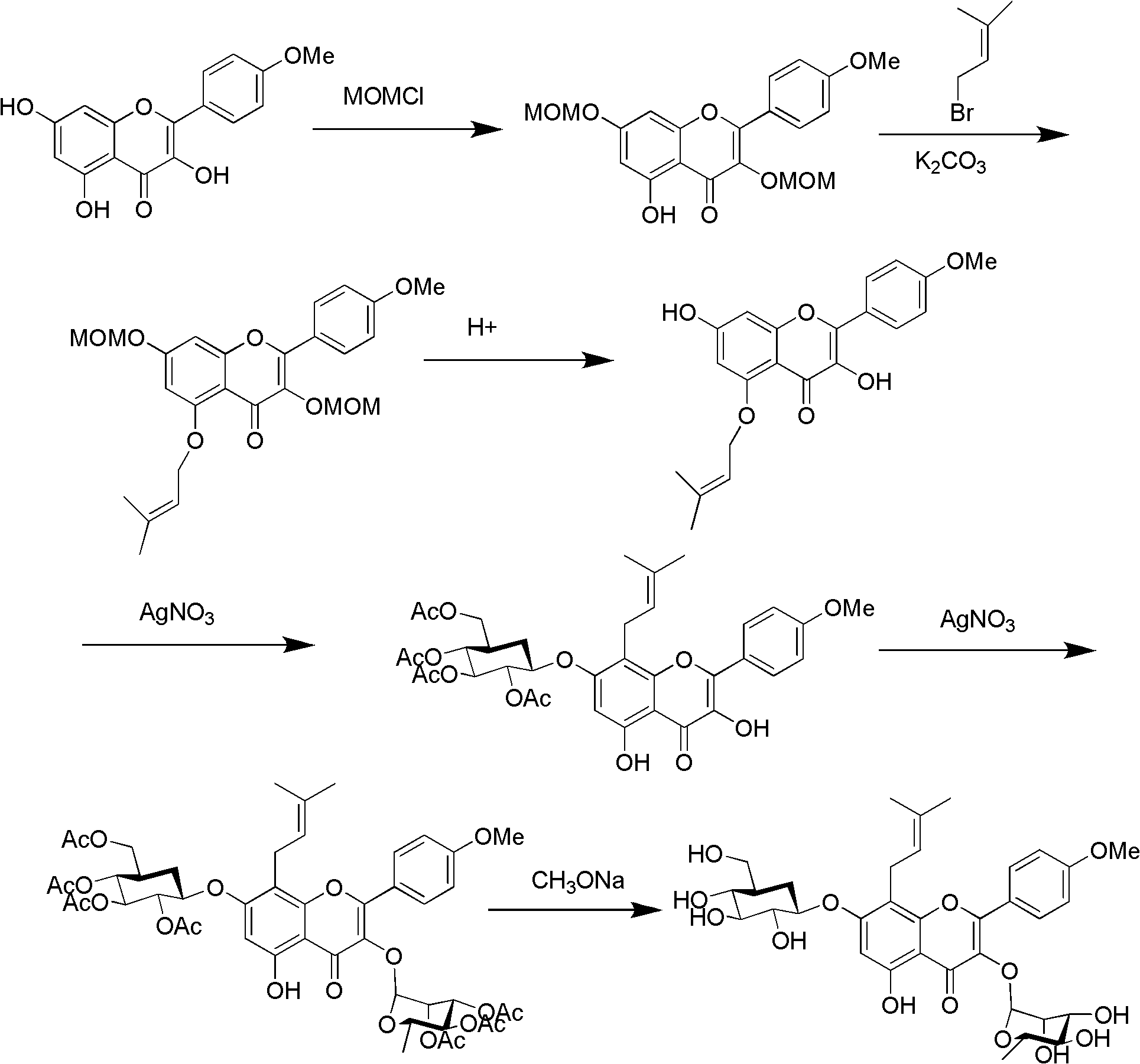
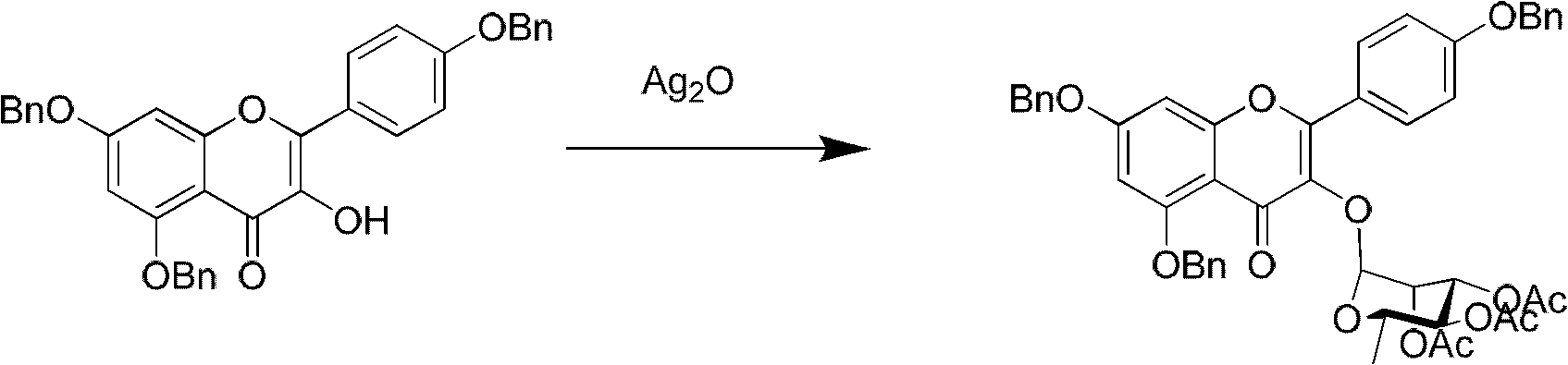Method for synthesizing icariin by glucosidation of dehydrated epimedium herb
A technology of icariin and chemical synthesis, which is applied in the direction of chemical instruments and methods, sugar derivatives, sugar derivatives, etc., can solve the problems of long reaction process, low yield, low sugar yield, etc., and achieve selective The effect of improving performance, enhancing selectivity and reducing cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (a) Put 200g of dry DMF into a 300ml reaction bottle, then add 20g (54.29mmol) of dehydrated icariin, stir and dissolve, add 18.4g (124.64mmol) of strontium carbonate, stir at room temperature for one hour, add α -Bromotetraacetyl-D-glucopyranose 24.6g (59.83mmol), continue to stir at room temperature for 48h, remove strontium bromide by filtration, concentrate the filtrate to dryness under reduced pressure, add 1000g of ethyl acetate, and then use 1% 500 g of hydrobromic acid aqueous solution was washed, washed with water, dehydrated with sodium sulfate and then concentrated to dryness. Column chromatography obtained 29.7 g of 7-O-β-tetraacetyl-D-glucopyranosyl anhydro-icarioside.
[0035] (b) Put 200g of dry dichloromethane into a 300ml reaction bottle, then add 29.7g of 7-O-β-tetraacetyl-D-glucopyranosyl dehydroicariside, stir to dissolve, then add Silver trifluoromethanesulfonate 10.8g and triethylamine 10ml (71.89mmol), after stirring at room temperature for one ho...
Embodiment 2
[0038] (a) Put 200g of dry acetonitrile into a 300ml reaction flask, then add 20g (54.29mmol) of dehydrated icariin, stir and dissolve, add 18.4g (124.64mmol) of strontium carbonate, and add α-bromotetraacetyl Base-D-glucopyranose 24.6g (59.83mmol), continued to stir at room temperature for 48h, filtered, the filtrate was concentrated to dryness under reduced pressure, added 1000g of ethyl acetate, then washed with 500g of 1% hydrobromic acid aqueous solution, washed with water, It was dehydrated with sodium sulfate and then concentrated to dryness. Column chromatography gave 20.9 g of 7-O-β-tetraacetyl-D-glucopyranosyl anhydro-icarioside.
[0039] (b) Put 200g of dry chloroform into a 300ml reaction bottle, then add 20.9g of 7-O-β-tetraacetyl-D-glucopyranosyl dehydroicariside, stir and dissolve, then add trifluoro Silver methanesulfonate 7.7g, stirred at room temperature for one hour, added 17.6g of α-bromotriacetyl-L-rhamnopyranose, continued to stir at room temperature for ...
Embodiment 3
[0042] (a) Put 200g of dry acetone into a 300ml reaction flask, then add 20g (54.29mmol) of dehydrated icariin, stir and dissolve, add 22.6g of strontium carbonate, add α-bromotetraacetyl-D- Glucopyranose 24.6g, stirred at 50°C for 48h, filtered, the filtrate was concentrated to dryness under reduced pressure, added 1000g of ethyl acetate, washed with water, dehydrated with sodium sulfate and concentrated to dryness, and obtained 7-O-β-tetraacetyl Base-D-glucopyranosyl dehydroicariside 27.9g.
[0043] (b) 200g of dry methylene chloride is dropped into a 300ml reaction flask, then 27.9g of 7-O-β-tetraacetyl-D-glucopyranosyl dehydroicariside is added, and after stirring and dissolving, three Silver fluoromethanesulfonate 12.8g and pyridine 8g, after stirring at room temperature for one hour, add α-bromotriacetyl-L-rhamnopyranose 18.2g, cool down to 0°C and stir for 24h, then remove silver bromide by filtration and pyridinium trifluoromethanesulfonate, the filtrate was concentra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com