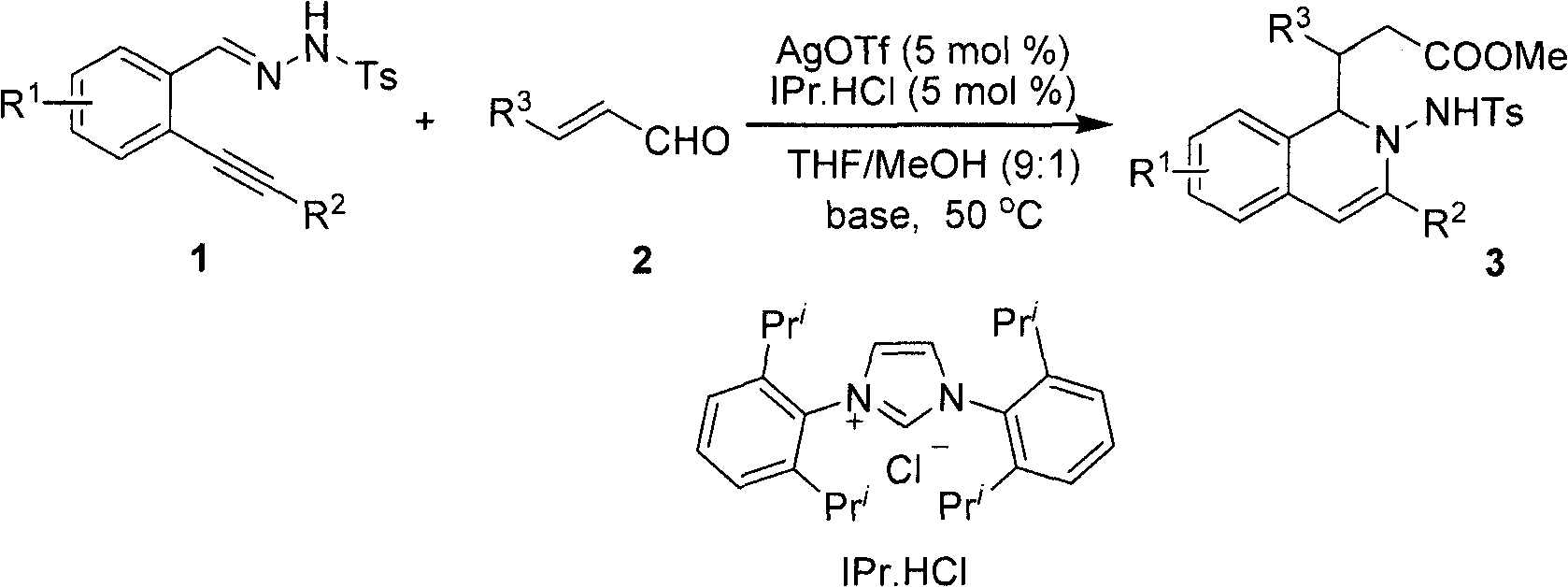
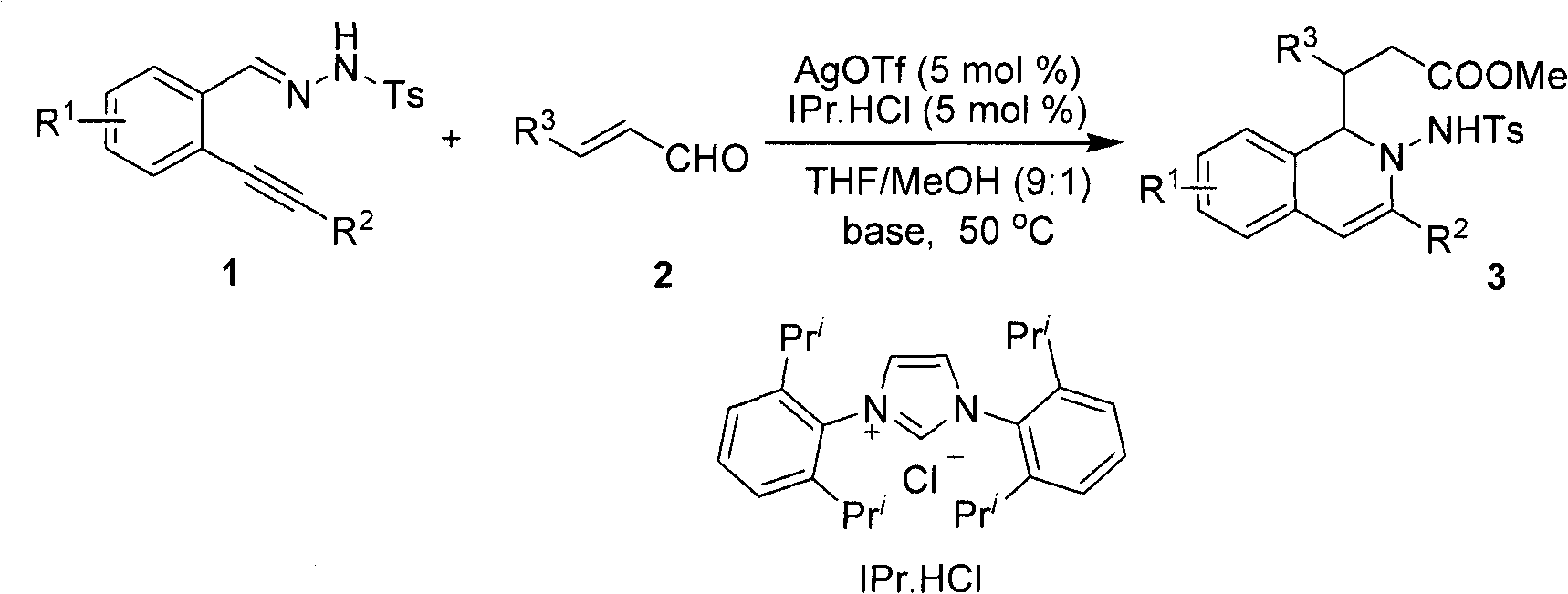
Method for preparing 2-amino-1,2-dihydro-isoquinoline compound
A technology of dihydroisoquinoline and compounds, applied in the direction of organic chemistry, can solve the problems of long synthetic route, low overall yield, and limited range of substrates, and achieve low cost, mild conditions, and easy separation The effect of purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018]
[0019] methyl
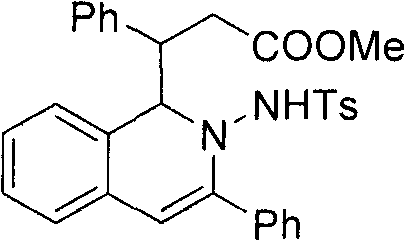
[0020] 3-(2-(4-methylphenylsulfonamido)-3-phenyl-1, 2-dihydroisoquinolin-1-yl)-3-phenylpropanoate (3a)
[0021] After dissolving o-phenylynyl benzylhydrazone (2mmol) and cinnamaldehyde (2.2mol%) in the mixed solvent THF / MeOH (20mL, v / v, 9:1), silver trifluoromethanesulfonate (5mol%) was added successively %) and nitrogen heterocyclic carbene catalyst (5mol%), cesium carbonate (25mol%), reacted for 12 hours at 50° C. under nitrogen protection, and TLC detected that the raw materials disappeared completely, and the reaction was completed. During the post-treatment, the solvent was first spin-dried, quenched with water, extracted with ethyl acetate, anhydrous MgSO 4 After drying, the solvent was evaporated to dryness under reduced pressure, and the pure product 2-amino-1,2-dihydroisoquinoline compound 3a was separated by silica gel column chromatography. Yield: 90%.
[0022] Yield: 94%; 1 H NMR (400MHz, CDCl3) 1H NMR (400MHz, CDCl3): 7.41-7.27 (m, ...
Embodiment 2
[0024]
[0025] methyl
[0026] 3-(4-bromophenyl)-3-(2-(4-methylphenylsulfonamido)-3-phenyl-1,2-dihydroisoquinolin-1-yl) propanoate (3b)
[0027] After dissolving o-phenylynylbenzylhydrazone (2.0mmol) and p-bromocinnamaldehyde (2.2mol%) in a mixed solvent THF / MeOH (20mL, v / v, 9:1), add trifluoromethanesulfonic acid in sequence Silver (5mol%), nitrogen heterocyclic carbene catalyst (5mol%), and potassium carbonate (25mol%) were reacted at 50° C. for 12 hours under nitrogen protection. TLC detected that the raw materials disappeared completely, and the reaction was completed. During the post-treatment, the solvent was first spin-dried, quenched with water, extracted with ethyl acetate, anhydrous MgSO 4 After drying, the solvent was evaporated to dryness under reduced pressure, and the pure product 2-amino-1,2-dihydroisoquinoline compound 3b was separated by silica gel column chromatography. Yield 78%.
[0028] 1 H NMR (400MHz, CDCl 3): δ7.51-7.47(m, 2H), 7.36-7.29(m, 2H)...
Embodiment 3
[0030]
[0031] methyl 3-(4-bromophenyl)-3-(3-(4-chlorophenyl)-2-(4-methylphenylsu-lfonamido)-1,2-dihydroisoquinolin-1-yl)propanoate(3c)
[0032] After dissolving o-4'-chlorophenylethynyl benzylhydrazone (2.0mmol) and p-bromocinnamaldehyde (2.2mol%) in the mixed solvent THF / MeOH (20mL, v / v, 9:1), add in sequence Silver triflate (5mol%), nitrogen heterocyclic carbene catalyst (5mol%), and cesium carbonate (25mol%) were reacted at 50° C. for 12 hours under nitrogen protection. TLC detected that the raw materials disappeared completely, and the reaction was completed. During the post-treatment, the solvent was first spin-dried, quenched with water, extracted with ethyl acetate, anhydrous MgSO 4 After drying, the solvent was evaporated to dryness under reduced pressure, and the pure product 2-amino-1,2-dihydroisoquinoline compound 3c was separated by silica gel column chromatography. Yield 82%.
[0033] 1 H NMR (400MHz, CDCl 3 ): δ7.51-7.47(m, 2H), 7.36-7.29(m, 2H), 7.22-7....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com