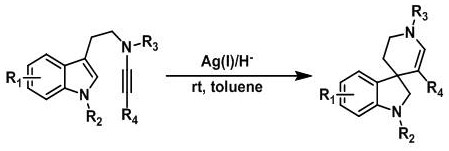
A kind of synthetic method of hydrogenated pyridine spiroindoline ring catalyzed by monovalent silver
A technology for hydrogenating a pyridine spiro indole ring and a synthesis method, which is applied in the field of medicine, can solve the problems of poor atom economy, expensive reagents, troublesome post-processing and the like, and achieves low price, short reaction time and simple post-processing. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Add substrate a1 (0.1mmol, 43mg) and Hans ester (0.2mmol, 51mg) in 25mL eggplant-shaped flask, add 5mL toluene solution, then add silver trifluoromethanesulfonate (0.01mmol, 2.6mg) in toluene The solution was 1 mL, stirred at room temperature, and stirred for 2.5 hours. After the reaction was completed, the target product b1 was separated by flash column chromatography (10:1 ratio of n-hexane: ethyl acetate), and the yield was 99%.
[0042] The reaction formula of Example 1 is:
[0043]
[0044] The spectral data of product b1 is: ESI-MS (m / z): 431[M+H] + ; 1 H-NMR (600MHz, DMSO) δ7.75 (d, J = 8.1Hz, 2H), 7.54 (d, J = 8.1Hz, 2H), 7.14–7.21 (m, 3H), 7.01 (td, J = 7.8 ,1.3Hz,1H),6.88(dd,J=7.8,1.3Hz,2H),6.86(s,1H),6.51(d,J=7.8Hz,1H),6.34(t,J=7.0Hz,1H ),5.86(d,J=7.0Hz,1H),3.62(dt,J=12.0,4.1Hz,1H),3.13(d,J=9.2Hz,1H),3.03–3.08(m,2H),2.61 (s,3H), 2.48(s,3H), 1.81–1.92(m,2H).
Embodiment 2
[0046] Add substrate a2 (0.1mmol, 45mg) and Hans ester (0.2mmol, 51mg) in 25mL eggplant-shaped flask, add 5mL toluene solution, then add silver trifluoromethanesulfonate (0.01mmol, 2.6mg) in toluene The solution was 1 mL, stirred at room temperature, and stirred for 2 hours. After the reaction was completed, the target product b2 was separated by flash column chromatography (n-hexane:ethyl acetate: 15:1), with a yield of 99%.
[0047] The reaction formula of Example 2 is:
[0048]
[0049] The spectral data of product b2 is: ESI-MS (m / z): 449[M+H] + ; 1H-NMR (600MHz, DMSO) δ7.74 (d, J = 8.2Hz, 2H), 7.51 (d, J = 8.2Hz, 2H), 6.97–7.02 (m, 3H), 6.87–6.90 (m, 2H ),6.82(s,1H),6.48(d,J=7.8Hz,1H),6.33(t,J=7.2Hz,1H),5.87(d,J=7.2Hz,1H),3.58(dt,J =12.1, 4.2Hz, 1H), 3.01–3.08(m, 3H), 2.59(s, 3H), 2.46(s, 3H), 1.77–1.89(m, 2H).
Embodiment 3
[0051] Add substrate a3 (0.1mmol, 44mg) and Hans ester (0.2mmol, 51mg) in 25mL eggplant-shaped flask, add 5mL toluene solution, then add silver trifluoromethanesulfonate (0.01mmol, 2.6mg) in toluene The solution was 1 mL, stirred at room temperature, and stirred for 2 hours. After the reaction was completed, the target product b3 was separated by flash column chromatography (n-hexane:ethyl acetate ratio of 10:1), with a yield of 99%.
[0052] The reaction formula of example 3 is:
[0053]
[0054] The spectral data of product b3 is: ESI-MS (m / z): 437[M+H] + ; 1 H-NMR (600MHz, DMSO) δ7.75 (d, J = 8.1Hz, 2H), 7.50 (d, J = 8.1, 2H), 7.23 (dd, J = 5.1, 1.0Hz, 1H), 7.12 (s ,1H),7.00–7.03(m,1H),6.82(dd,J=5.1,3.6Hz,1H),6.55(dd,J=3.6,1.0Hz,1H),6.49(d,J=7.8Hz, 1H), 6.44(t, J=7.2Hz, 1H), 6.26(d, J=7.2Hz, 1H), 3.43–3.49(m, 1H), 3.32–3.37(m, 1H), 3.15(q,J =9.4Hz, 2H), 2.62(s, 3H), 2.43(s, 3H), 1.94(ddd, J = 13.6, 6.8, 2.8Hz, 1H), 1.51–1.57(m, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com