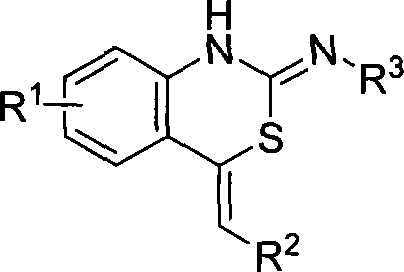
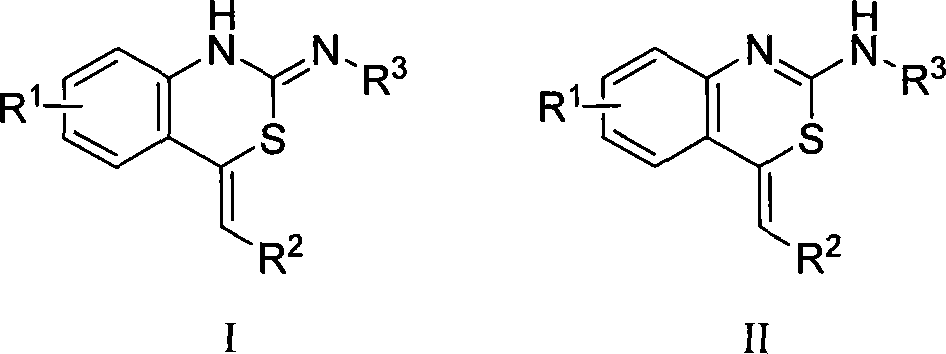
Method for producing benzo thiazides compounds
A technology of benzothiazine and compound, which is applied in the field of preparation of benzothiazine compounds, and achieves the effects of good application prospect, high product yield, and simple reaction operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Example 1 2-(2-phenylethynyl)aniline (193.0mg, 1.0mmol), phenylisothiocyanate (405.0mg, 3.0mmol), silver trifluoromethanesulfonate (2.6mg, 0.01mmol) , tetrahydrofuran (2 mL) was stirred at room temperature for about 24 hours. The solvent was evaporated to dryness, water (10 mL) was added, extracted with ethyl acetate (20 mL×2), dried over anhydrous sodium sulfate, concentrated and separated by column chromatography to obtain 321 mg of a colorless substance with a yield of 96%.
[0016] 1 H NMR (400MHz, CDCl 3 )δ7.06(t, J=7.3Hz, 1H), 7.17(s, 1H), 7.19(dd, J=1.5, 7.8Hz, 1H), 7.27-7.40(m, 5H), 7.41-7.45(m , 4H), 7.53-7.58 (m, 3H); 13 C NMR (100MHz, CDCl 3 )δ120.2, 121.7, 123.5, 124.8, 124.9, 125.9, 126.6, 127.1, 127.7, 128.2, 128.9, 129.3, 129.6, 135.6, 139.9, 143.2, 147.6; Ms (EI) m / z 328 (M + ); Elemental analysis calcd(%) for C 21 h 16 N 2 S: C 76.80, H 4.91, N 8.53; Found: C 76.64, H 4.92, N 8.62.
Embodiment 2
[0018] 2-(2-Phenylethynyl)aniline (92.0mg, 0.5mmol), 4-nitro-phenylisothiocyanate (180.0mg, 2.0mmol), silver trifluoromethanesulfonate (6.5mg, 0.025 mmol), tetrahydrofuran (1 mL) was stirred at room temperature for about 18 hours. The solvent was evaporated to dryness, water (10 mL) was added, extracted with ethyl acetate (20 mL×2), dried over anhydrous sodium sulfate, concentrated and separated by column chromatography to obtain 183 mg of a colorless substance with a yield of 98%.
[0019] 1 H NMR (400MHz, DMSO) δ7.18-7.50(m, 10H), 7.65(d, J=7.3Hz, 1H), 8.02(d, J=8.0Hz,, 2H), 8.13(d, J=8.3 Hz, 2H); 13 C NMR (100MHz, DMSO) δ119.2, 121.3, 125.4, 125.8, 126.4, 127.3, 128.3, 128.8, 129.7, 130.4, 135.8, 141.7, 142.9, 146.7, 147.3; Ms (EI) m / z 373 (M + ); Elemental analysis calcd(%) for C 21 h 15 N 3 o 2 S: C 67.54, H 4.05, N 11.25; Found: C 67.82, H 3.98, N 10.97.
Embodiment 3
[0021] 4-Methyl-2-(2-phenylethynyl)aniline (103.6mg, 0.5mmol), phenylisothiocyanate (135.0mg, 1.0mmol), silver trifluoromethanesulfonate (6.5mg, 0.025 mmol), tetrahydrofuran (2 mL) was stirred at room temperature, and TLC tracked and detected that the reaction was complete for 36 hours. The solvent was evaporated to dryness, water (10 mL) was added, extracted with ethyl acetate (20 mL×2), dried, concentrated and separated by column chromatography to obtain 167.6 mg of a colorless substance with a yield of 98%.
[0022] 1 H NMR (400MHz, CDCl 3 )δ2.38(s, 3H), 7.04(t, J=7.3Hz, 1H), 7.13-7.17(m, 3H), 7.27-7.33(m, 3H), 7.35(s, 1H), 7.38-7.43 (m, 4H), 7.53 (d, J=8.3Hz, 2H); 13 C NMR (100MHz, CDCl 3 )δ20.5, 119.5, 120.8, 122.8, 124.5, 125.3, 126.1, 126.3, 127.0, 127.6, 128.4, 128.8, 130.0, 133.9, 135.2, 139.4, 140.4, 146.4; Ms(EI) m / z 328(M + );Elemental analysis calcd(%)forC 21 h 16 N 2 S: C 76.80, H 4.91, N 8.53; Found: C 76.65, H 4.90, N 8.35.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com