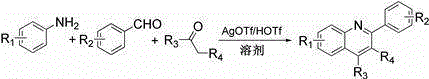
Method for synthesizing quinoline derivative by utilizing arylamine, aromatic aldehyde and ketone
A technology of aromatic amines and aromatic aldehydes, applied in the field of synthesis of quinoline derivatives, can solve problems such as limited quinoline derivatives, complex reaction steps, and poor reaction effects, and achieve easy separation and purification, efficient preparation, and simple operation steps Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 2, the synthetic method of 4-diphenylquinoline is as follows: in reaction vessel, add aniline 0.5mmol (46.5mg), benzaldehyde 0.5mmol (53mg), acetophenone 0.75mmol (90.1mg), catalyst AgOTf0.005mmol ( 1.29mg), HOTf0.01mmol (1.5mg), toluene 2mL. React in an oil bath at 120°C for 24 hours, cool to room temperature, add water to quench the reaction, wash three times with ethyl acetate, separate the layers, combine the organic layers, decolorize with activated carbon, filter, dry over anhydrous sodium sulfate, concentrate under reduced pressure, and the product passes through Purified by column chromatography, the eluent was petroleum ether: ethyl acetate = 10:1 (v / v), to obtain a white solid product with a yield of 94% and a purity of 99.9%. 1 HNMR (500MHz, CDCl 3 ) ppm: 8.43 (d, J =8.0Hz, 1H), 8.34(d, J =8.0Hz, 2H), 8.00(d, J =8.5Hz, 1H), 7.92(s, 1H), 7.80(t, 1H), 7.51-7.64(m, 9H); 13 CNMR (500MHz, CDCl 3 ): 156.90, 149.23, 149.05, 139.77, 138.56, 130.35, 129.70, 129....
Embodiment 2
[0031] The synthetic method of 6-trifluoromethyl-2,4-diphenylquinoline is as follows: in reaction vessel, add p-trifluoromethylaniline 0.5mmol (80.6mg), benzaldehyde 0.5mmol (53mg), acetophenone 0.75mmol (90.1mg), catalyst AgOTf0.005mmol (1.29mg), HOTf0.01mmol (1.5mg), toluene 2mL. React in an oil bath at 120°C for 24 hours, cool to room temperature, add water to quench the reaction, wash three times with ethyl acetate, separate the layers, combine the organic layers, decolorize with activated carbon, filter, dry over anhydrous sodium sulfate, concentrate under reduced pressure, and the product passes through Purified by column chromatography, the eluent was petroleum ether: ethyl acetate = 5:1 (v / v), to obtain a white solid product with a yield of 82% and a purity of 99.8%. 1 HNMR (400MHz, CDCl 3 ) δ ppm: 8.32 (d, J =8.8Hz, 1H), 8.20(t, 3H), 7.87(d, J=8.0Hz, 2H), 7.44-7.55(m, 8H); 13 CNMR (100MHz, CDCl 3 ) δ ppm: 158.9, 150.2, 150.0, 139.0, 137.5, 131.4, 130.1, 129.6, ...
Embodiment 3
[0033] 6-methyl-2, the synthetic method of 4-diphenylquinoline is as follows: in reaction vessel, add p-methylaniline 0.5mmol (53.5mg), benzaldehyde 0.5mmol (53mg), acetophenone 0.75mmol (90.1 mg), catalyst AgOTf0.005mmol (1.29mg), HOTf0.01mmol (1.5mg), toluene 2mL. React in an oil bath at 120°C for 24 hours, cool to room temperature, add water to quench the reaction, wash three times with ethyl acetate, separate the layers, combine the organic layers, decolorize with activated carbon, filter, dry over anhydrous sodium sulfate, concentrate under reduced pressure, and the product passes through Purified by column chromatography, the eluent was petroleum ether: ethyl acetate = 10:1 (v / v), to obtain a white solid product with a yield of 96% and a purity of 99.9%. 1 HNMR (500MHz, CDCl 3 ) ppm: 8.85 (d, J =9.0Hz, 1H), 8.15(t, 2H), 7.97(d, J =3.5Hz, 2H), 7.88(s, 1H), 7.62-7.73(m, 8H), 2.61(s, 3H); 13 CNMR (500MHz, DMSO- d 6 ): 155.7, 153.4, 140.0, 139.4, 136.2, 136.1, 132.9, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com