Synthetic method of 3-methylamino-1-(2-thienyl)-1-acetone hydrochloride
A technology of acetone hydrochloride and a synthesis method, applied in the field of pharmaceutical synthesis, can solve problems such as unfavorable market competition, high production cost, high environmental protection pressure, etc., and achieve the effects of simple and convenient post-processing, low environmental protection pressure, and low equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
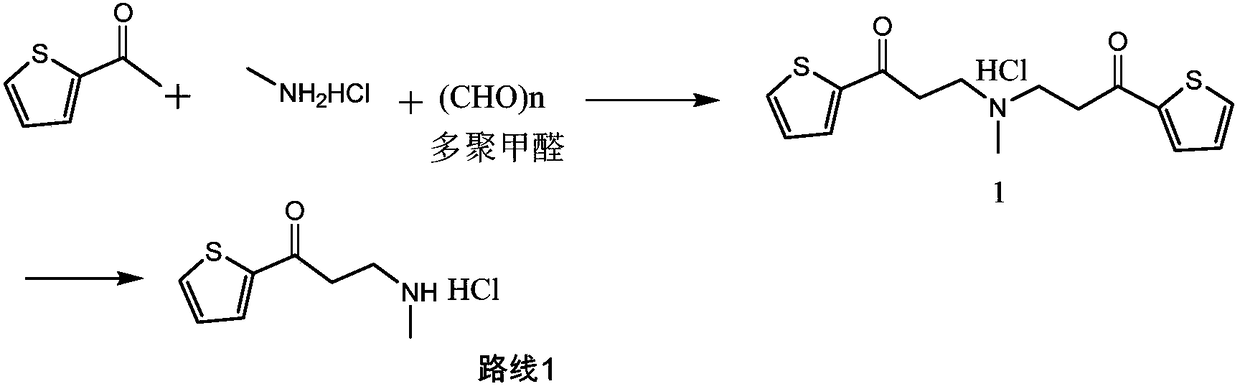
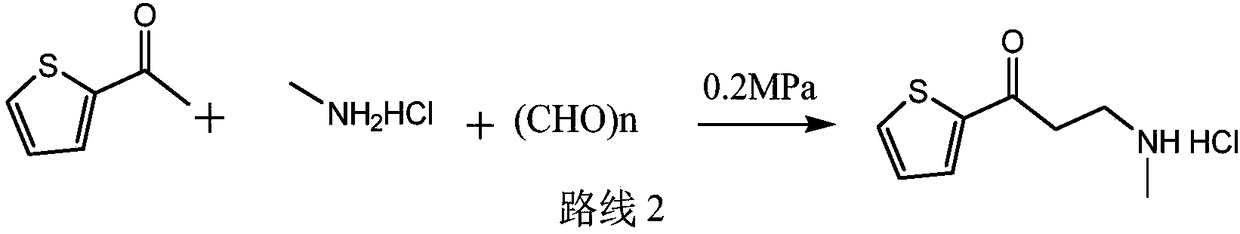
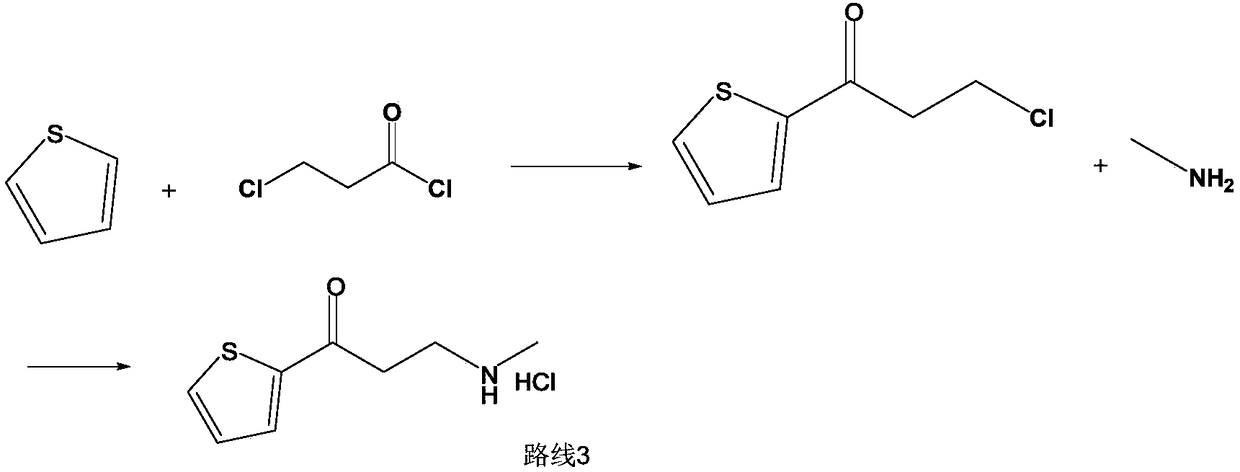
[0028] At room temperature, add 2-acetylthiophene (126g, 1mol), methylamine hydrochloride (74.3g, 1.1mol), 37% aqueous formaldehyde (90g, 1.1mol), and 150ml of ethanol into a 1L reaction flask, and dissolve the solid , add 0.3g of silver trifluoromethanesulfonate, control the reaction pressure to normal pressure, and the reaction temperature is 25°C to react for 6 hours. If the residual amount of 2-acetylthiophene in HPLC is not more than 2%, it is judged that the reaction is over, otherwise the reaction is prolonged time to the end of the reaction.
[0029] At the end of the reaction, the reaction solution was concentrated to dryness under reduced pressure, 600ml of toluene was added, the crystals were dispersed at 20-25°C for 2 hours, filtered, rinsed with 50ml of toluene, and the obtained wet product was dried to obtain 170.5g of the compound of formula (I), with a yield of 83 %. 1 H NMR (400MHz, DMSO) δ9.25(s, 2H), 8.08(dd, J=4.9, 1.1Hz, 1H), 8.01(dd, J=3.8, 1.1Hz, 1H), 7...
Embodiment 2
[0031] At room temperature, add 2-acetylthiophene (126g, 1mol), methylamine hydrochloride (74.3g, 1.1mol), 37% formaldehyde aqueous solution (90g, 1.1mol), purified water 125ml, and dissolve the solid in a 1L reaction flask. Clear, add 0.3g of silver trifluoromethanesulfonate, control the reaction pressure to normal pressure, and the reaction temperature is 25 ℃ for 6 hours, if the residual amount of 2-acetylthiophene in HPLC is not more than 2%, it is judged that the reaction is over, otherwise the extension Reaction time to the end of the reaction.
[0032] At the end of the reaction, the reaction solution was concentrated to dryness under reduced pressure, 600ml of toluene was added, the crystals were dispersed at 20-25°C for 2 hours, filtered, rinsed with 50ml of toluene, and the obtained wet product was dried to obtain 182.5g of the compound of formula (I), with a yield of 89% %.
Embodiment 3
[0034] At room temperature, add 2-acetylthiophene (126g, 1mol), methylamine hydrochloride (74.3g, 1.1mol), 37% aqueous formaldehyde (90g, 1.1mol), and 150ml of ethanol into a 1L reaction flask, and dissolve the solid , add 0.3 g of indium trichloride, control the reaction pressure to normal pressure, and react at 25°C for 6 hours. If the residual amount of 2-acetylthiophene in HPLC does not exceed 2%, it is judged that the reaction is over, otherwise the reaction time is extended to The reaction is over.
[0035] At the end of the reaction, the reaction solution was concentrated to dryness under reduced pressure, and 600ml of toluene was added, and the crystals were dispersed at 20-25°C for 2 hours, filtered, rinsed with 50ml of toluene, and the obtained wet product was dried to obtain 172.5g of the compound of formula (I), with a yield of 83.9 %.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com