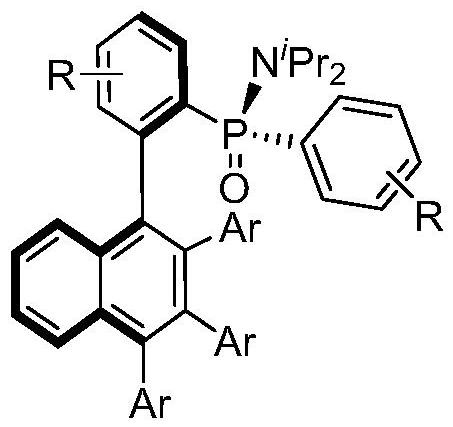
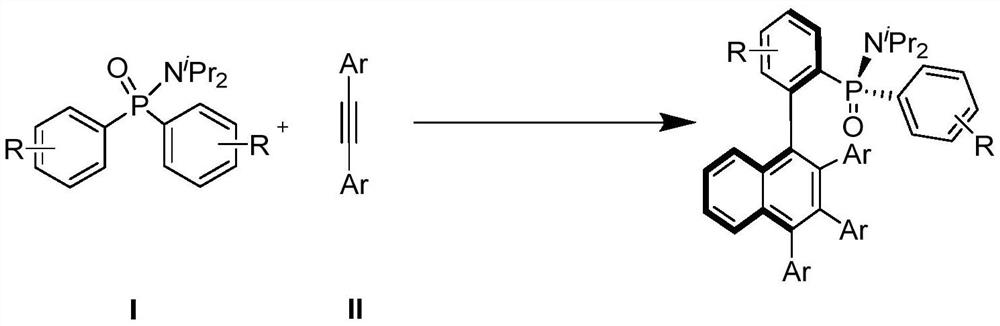
Axially chiral biaryl compound with P-stereo center and synthesis method and application thereof
A synthesis method and compound technology, which can be used in organic chemistry methods, preparation of organic compounds, organic compounds/hydrides/coordination complex catalysts, etc. The effect of enantioselectivity, mild reaction conditions, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Synthesis of (S)-N,N-diisopropyl-P-phenyl-P-((R))-2-(2,3,4-triphenyl-1-naphthyl)phenyl with the following structural formula ) Phosphinamide
[0027]
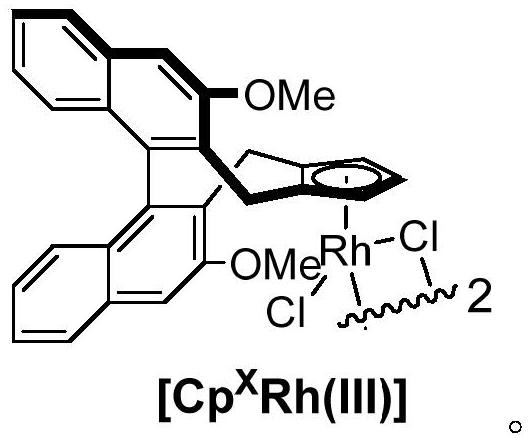
[0028] Under nitrogen atmosphere, add 30.1mg (0.1mmol) N,N-diisopropyl-P,P-diphenylphosphinamide, 44.6mg (0.25mmol) toluene, 4.6mg ( 0.004mmol) [Cp X Rh(III)], 4.1mg (0.016mmol) silver trifluoromethanesulfonate, 41.8mg (0.25mmol) silver acetate, 25.6mg (0.2mmol) cyclohexyl formic acid, 2mL dichloromethane, tighten the pressure tube, 60℃ The reaction was stirred for 24 hours. After the reaction was completed, the dichloromethane was removed by rotary evaporation under reduced pressure to obtain a crude product. The crude product was passed through a column with silica gel (petroleum ether:ethyl acetate=10:1~5:1) to obtain a light yellow solid product , and its yield is 71% (yield is calculated based on phosphoramide), and the characterization data are: 1 H NMR (600MHz, CDCl 3 )δ8.05–7.97(m,1H),7.63–7.54(m,3H),7.46–...
Embodiment 2
[0030] Synthesis of (S)-N,N-diisopropyl-P-((R))-4-methoxy-2-(2,3,4-triphenyl-1-naphthyl)benzene with the following structural formula base)-P-(4-methoxyphenyl)phosphinamide
[0031]
[0032] In this example, the N,N-diisopropyl group used in Example 1 was replaced with equimolar N,N-diisopropyl-P,P-bis(4-methoxyphenyl)phosphinamide -P, P-diphenylphosphinamide, other steps are the same as in Example 1, to obtain a light yellow solid product, the yield is 60%, and the characterization data are: 1 H NMR (600MHz, CDCl 3 )δ7.92(dd,J=11.5,8.9Hz,1H),7.52–7.43(m,4H),7.35–7.27(m,3H),7.24–7.17(m,2H),7.16–7.14(m, 2H),6.92(d,J=8.7Hz,1H),6.88–6.74(m,6H),6.74–6.64(m,5H),6.47(d,J=7.5Hz,1H),6.28(t,J =7.5Hz,1H),3.78(s,3H),3.69(s,3H),3.44–3.51(m,2H),1.13(d,J=6.7Hz,6H),1.05(d,J=6.7Hz ,6H); 13 C NMR (151MHz, CDCl 3 )δ161.1(d, J=2.8Hz), 160.4(d, J=2.7Hz), 146.1(d, J=11.4Hz), 140.9, 140.3, 139.7, 138.5, 138.2, 138.0, 138.0, 137.1, 135.0 (d,J=9.5Hz),134.2(d,J=10.9Hz),132.9,131.7,131.7,131....
Embodiment 3
[0034] Synthesis of (S)-P-((R)-4-fluoro-2-(2,3,4-triphenyl-1-naphthyl)phenyl)-P-(4-fluorophenyl) with the following structural formula -N,N-Diisopropylphosphinamide
[0035]
[0036] In this example, N,N-diisopropyl-P,P-bis(4-fluorophenyl)phosphinamide used in Example 1 was replaced with equimolar N,N-diisopropyl-P , P-diphenylphosphinamide, equimolar silver hexafluoroantimonate instead of silver trifluoromethanesulfonate used in Example 1, other steps are the same as Example 1, to obtain light yellow solid product, its yield is 80%, the characterization data is: 1 H NMR (600MHz, CDCl 3 )δ8.02–7.98(m,1H),7.55(d,J=8.0Hz,1H),7.53–7.48(m,2H),7.40–7.37(m,2H),7.36–7.34(m,2H) ,7.32–7.29(m,1H),7.21–7.17(m,2H),7.18–7.13(m,1H),7.10–7.04(m,2H),6.94–6.83(m,6H),6.83–6.77( m,2H),6.73(t,J=7.3Hz,1H),6.70–6.66(m,1H),6.49(d,J=7.6Hz,1H),6.35(t,J=7.4Hz,1H), 3.46–3.39(m,2H),1.14(d,J=6.7Hz,6H),1.07(d,J=6.8Hz,6H); 13 C NMR (151MHz, CDCl 3 )δ164.1 (dd, J=3.3, 251.8Hz), 163.3 (dd, J=3.2, 25...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com