In-situ nitrogen doped graphalkyne material and synthesizing and application methods thereof
A synthesis method, nitrogen doping technology, applied in the direction of hybrid capacitor electrodes, nano-carbon, etc., can solve the limitations of in-depth research and application of nitrogen-doped graphyne materials, nitrogen doping amount, position and type can not be adjusted and affected Intrinsic properties of graphyne and other issues, to achieve the effect of good chemical and thermal stability, strong controllability and adjustability, and good purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
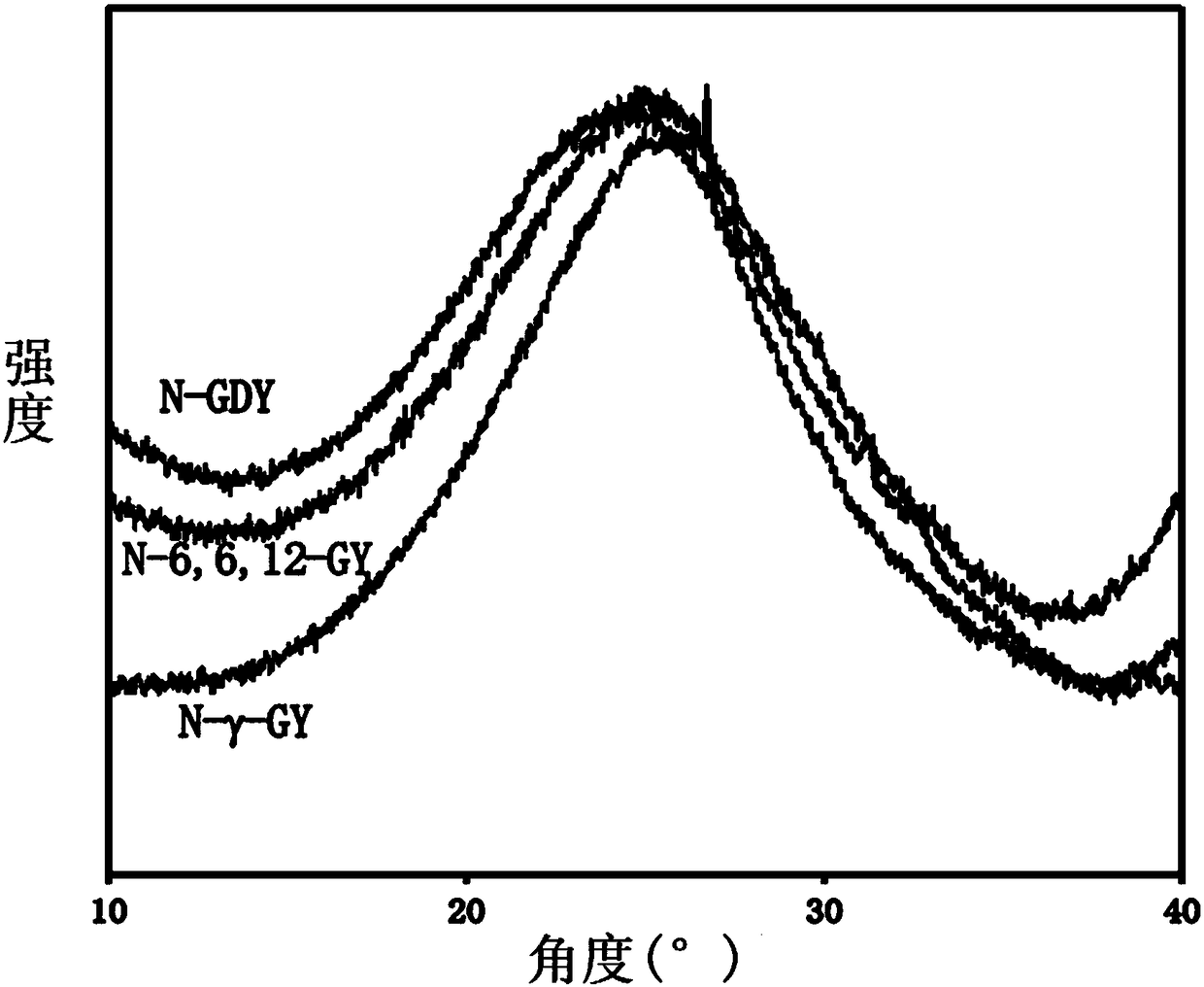
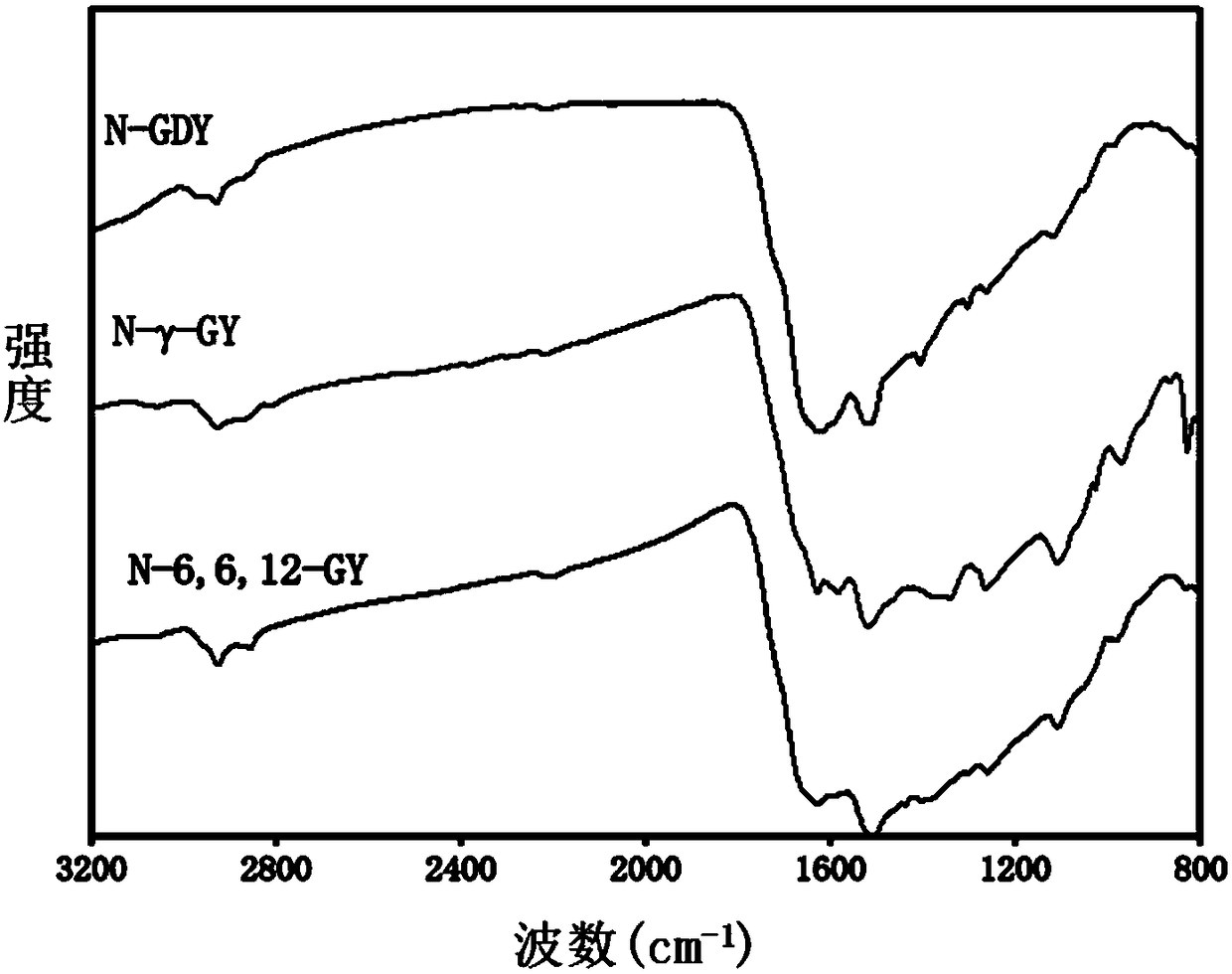
[0068] The synthesis of embodiment 1 aza-gamma-graphyne (N-gamma-GY):
[0069] Under argon protection, 2,4,6-triethynyl-1,3,5-triazine (76.5mg, 0.5mmol), Pd(PPh 3 ) 4 (Tetrakis(triphenylphosphine)palladium 28.85mg, 0.025mmol), 2,4,6-trichloro-1,3,5-triazine (92mg, 0.5mmol) and CuCl (5mg, 0.05mmol) were added to 500mL In a three-necked flask, 30 mL of N,N-dimethylformamide was added to the mixture. The reaction was stirred at 120° C. for 3 days and then cooled to room temperature. The insoluble solid was obtained by suction filtration, and then fully washed with water, ethanol, methyl alcohol, acetone and chloroform (to remove unreacted monomers, oligomers, catalysts and by-products) ), and then extracted with tetrahydrofuran, methanol, and chloroform for 24 hours, and finally vacuum-dried at 100° C. for 12 hours to obtain a black solid powder (101 mg, yield 88%). The specific surface area of the obtained aza-γ-graphyne is 837m 2 / g, the pore volume is 1.45cm 3 / g, it ha...
Embodiment 2
[0070] The synthesis of embodiment 2 aza 6,6,12 graphyne (N-6,6,12-GY):
[0071] Under argon protection, 2,4,6-triethynyl-1,3,5-triazine (76.5mg, 0.5mmol), Pd(PPh 3 ) 4 (28.85mg, 0.025mmol) and CuCl (5mg, 0.05mmol), then 30mL of N,N-dimethylformamide was added, and tetrachloroethylene (0.038ml, 0.375mmol) was added dropwise. The reaction was stirred at 120°C for three days and then cooled to room temperature. After the insoluble solid was obtained by suction filtration, it was fully washed with water, ethanol, methanol, acetone and chloroform, then extracted with tetrahydrofuran, methanol, and chloroform for 24 hours, and finally vacuum-dried at 100°C for 12 hours to obtain a black solid powder (101mg, 118 %). The specific surface area of the obtained aza 6,6,12 graphyne is 727m 2 / g, the pore volume is 1.23cm 3 / g, it has a micropore diameter of 1.3nm, a mesopore diameter of 2-9nm, and excellent comprehensive properties.
Embodiment 3
[0072] Synthesis of embodiment 3 azagraphdiyne (N-GDY):
[0073] Under argon protection, 2,4,6-triethynyl-1,3,5-triazine (76.5mg, 0.5mmol) and CuCl (50mg, 0.5mmol) were added to a 500ml three-necked flask, and then Add 45 mL of N,N-dimethylformamide. The reaction was stirred at 120°C for 3 days, then cooled to room temperature, and the insoluble solid was obtained by suction filtration, washed thoroughly with dilute hydrochloric acid, ethanol, methanol, acetone and chloroform, then extracted with tetrahydrofuran, methanol, and chloroform for 24 hours, and finally 100°C Drying in vacuo for 12 hours gave a black solid powder (77 mg, 103%). The specific surface area of the obtained azagraphdiyne is 683m 2 / g, the pore volume is 1.89cm 3 / g, it has a micropore diameter of 1.8nm, a mesopore diameter of 3-8nm, and excellent comprehensive properties.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Pore volume | aaaaa | aaaaa |
| Pore diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com