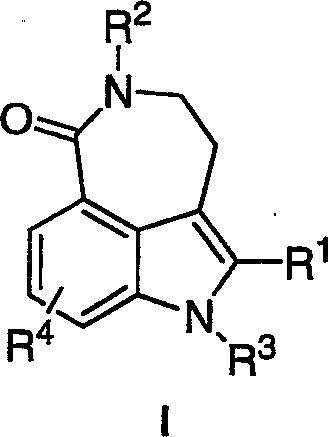
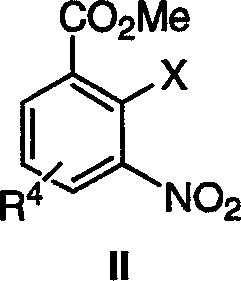
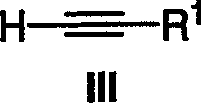Method of preparing poly(ADP-ribose) polymerases inhibitors
A compound and alkyl technology, applied in the field of preparing compounds that inhibit polymerase, can solve the problem of no PARP inhibitor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Example 1. Synthesis of 4-trimethylsilylethynyl-benzaldehyde (2)
[0083]
[0084] 4-Bromobenzaldehyde (1) (185g, 1.0mol) was dissolved in THF (1L), then copper(I) iodide (7.6g, 0.04mol), dichlorobis(triphenylphosphine)palladium was added (II) (14.02 g, 0.02 mol) and triethylamine (151.5 g, 1.5 mol). Ethynyltrimethylsilane (109.1 g, 1.11 mol) was added from the addition funnel as a solution in THF (0.2 L). The reaction was stirred at 30°C for 30 minutes, then at 25°C for 20 hours. Analysis by HPLC indicated the reaction was complete. THF was removed and the residue was treated with hexanes (1.8 L). The solids were removed by filtration, and the filter cake was washed with hexane (0.3 L). The combined hexane solutions were washed with water (2 x 0.5 L). Remove hexane in rotovap. The residue was dissolved in EtOH (0.5 L) at 50 °C. The solution was then cooled slowly to 16°C and stirred for 30 minutes. The product started to crystallize. The mixture was furth...
Embodiment 2
[0085] Example 2. Synthesis of methyl-(4-trimethylsilylethynyl-benzyl)-amine (3)
[0086]
[0087]Methylamine (8M / MeOH, 135ml) and methylamine hydrochloride (44.0g, 0.65mol) were dissolved in methanol (900ml). Add aldehyde 2 (44.0 g, 0.22 mol) and stir at room temperature for 30 minutes. Sodium cyno borohydride (17.42 g, 0.28 mol) was then added. After the addition was complete, hydrochloric acid in methanol was added to adjust the pH to 5 while maintaining the temperature at ~30°C. The reaction was stirred for 2 hours. The pH of the reaction mixture was maintained at 4-6 by addition of hydrochloric acid / MeOH solution. The reaction solvent was removed. The residue was mixed with water (400ml) and brine (50ml). The mixture was extracted with dichloromethane (2 x 300ml). The combined organic solutions were concentrated to dryness to give the crude product (40.6 g, ~85% yield), which was used in the next step without further purification. 1 H NMR (300MHz, CDCI3) δ0.25...
Embodiment 3
[0088] Example 3. Synthesis of methyl-(4-trimethylsilylethynyl-benzyl)-methyl carbamate (4)
[0089]
[0090] Amine 3 (90.0 g, -0.41 mol) was dissolved in dichloromethane (810 ml). Triethylamine (66.6 g, 0.66 mol) was added and the solution was cooled to 5°C. Then methyl chloroformate (47.0 g, 0.50 mol) / dichloromethane (100 ml) was added slowly, and the reaction temperature was kept at 10°C-14°C. After the addition was complete, the reaction solution was stirred at room temperature for 12 hours. Water (540ml) was added. The aqueous phase is separated. The organic phase was concentrated to dryness to afford crude 4. The crude product was used in the next step without further purification. 1 H NMR (300MHz, CDCl 3 )δ 0.279(s, 9H), 2.86(d, br, 3H), 3.774(s, 3H), 4.484(s, br, 2H), 7.190(s, br, 2H), 7.46(d, 2H, J = 8.10Hz).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com