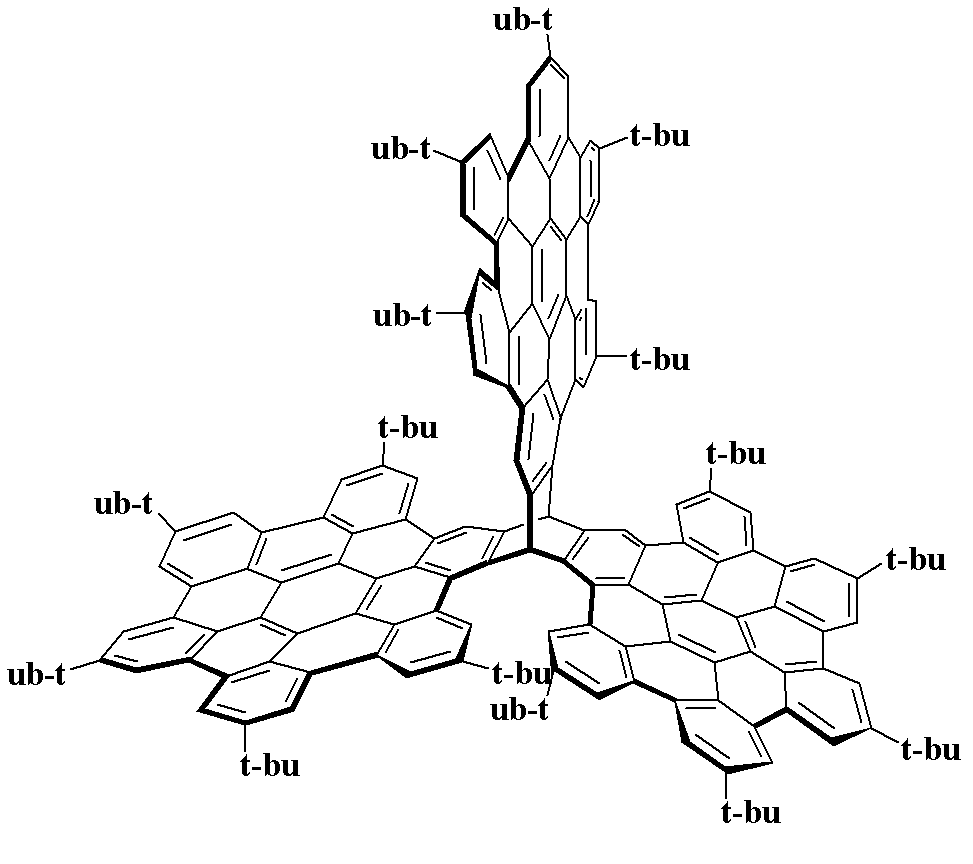
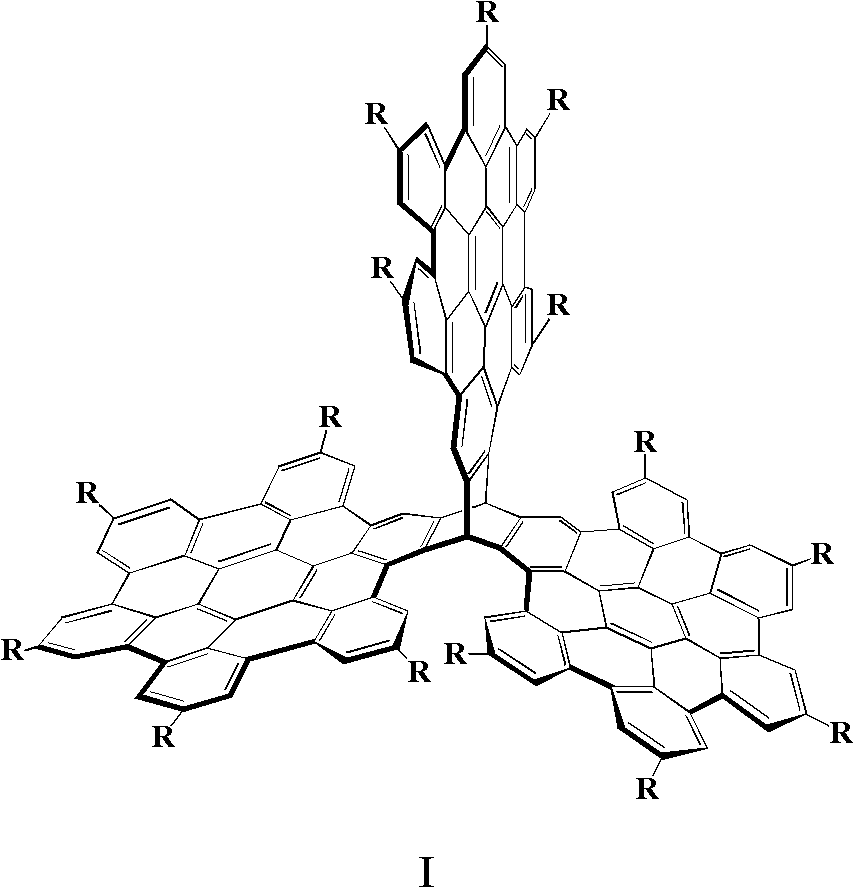
Three-dimensional nanometer graphene based on triptycene and preparation method thereof
A three-dimensional nano, triptycene technology, applied in the field of chemistry, can solve the problems that have not been reported, and achieve the effects of novel structure, strong fluorescence performance and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Synthesis of 2,6,14-triphenylethynyl triptycene
[0032] 200mg triiodotriptycene (0.315mmol), 37mg Pd(PPh 3 ) 4 (0.036mmol) and 11.6mgCuI (0.061mmol) were added in a 100ml two-necked bottle, and argon was ventilated, and 200ul phenylacetylene (0.99mmol) and 40ml triethylamine were added, then stirred and refluxed at 70°C, and the reaction was stopped after 48 hours. Add dichloromethane to dissolve, successively wash with dilute hydrochloric acid solution, water, extract with dichloromethane, anhydrous Na 2 SO 4 After drying, column chromatography using dichloromethane-petroleum ether (1:3) as the eluent gave 2,6,14-triphenylethynyl triptycene as a white solid.
[0033] Yield: 68.0%. Melting point: 184-185°C
[0034]
[0035] 1 H NMR (400MHz, CDCl 3 ): δ5.45(s, 1H), 5.46(s, 1H), 7.25(dd, J=7.6, 1.2Hz, 3H), 7.33-7.37(m, 9H), 7.40(d, J=7.6Hz, 3H), 7.50-7.53(m, 6H), 7.60(d, J=1.2Hz, 3H). 13 CNMR (100MHz, CDCl 3 ): δ53.41, 53.50, 88.77, 89.40, 120.39, 1...
Embodiment 2
[0036] Example 2 Synthesis of 2,6,14-tri-tert-butylphenylethynyl triptycene
[0037] 200mg triiodotriptycene (0.315mmol), 37mg Pd(PPh 3 ) 4 (0.036mmol) and 11.6mgCuI (0.061mmol) were added to a 100ml two-necked flask, argon was introduced, 178ul p-tert-butylphenylacetylene (0.99mmol) and 40ml triethylamine were added, and then stirred and refluxed at 70°C for 24 hours Then stop the reaction, add dichloromethane to dissolve, wash with dilute hydrochloric acid solution, water, extract with dichloromethane, anhydrous Na 2 SO 4 After drying, column chromatography with dichloromethane-petroleum ether (1:10) was used as the eluent to obtain 2,6,14-tri-tert-butylphenylethynyltriptycene as a white solid product.
[0038] Yield: 70.4%. Melting point: 190-193°C
[0039]
[0040] 1 H NMR (400MHz, CDCl 3 ): δ1.310(s, 9H), 5.404(s, 1H), 5.413(s, 1H), 7.210(dd, J=2.8, 2.8Hz, 3H), 7.335-7.355(m, 9H), 7.421( d, J=8HZ, 6H), 7.556(s, 3H). 13 C NMR (100MHz, CDCl 3): δ29.50, 29.67, 30...
Embodiment 3
[0041] Example 3 Synthesis of 2,6,14-three (2,3,4,5,6-pentaphenyl)-phenyl triptycene
[0042] 100mg of triphenylethynyl triptycene (0.181mmol), 215mg of tetraphenylcyclopentadienone (0.560mmol) and 1.5ml of diphenyl ether were heated and stirred at 260°C under the protection of argon to react after 48 hours completely. After column chromatography, dichloromethane-petroleum ether (1:3) was used as the eluent to obtain a yellow solid 2,6,14-tris(2,3,4,5,6-pentaphenyl)-phenyltris pterene.
[0043] Yield: 52.0%. Melting point: >300°C
[0044]
[0045] 2,6,14-tris(2,3,4,5,6-pentaphenyl)-phenyl triptycene
[0046] 1 H NMR (400MHz, CDCl 3 ): δ4.05(s, 1H), 4.28(s, 1H), 6.29-6.36(m, 9H), 6.48-6.61(m, 9H), 6.72-6.82(m, 66H). 13 C NMR (100MHz, CDCl 3 ):δ52.79,53.00,121.63,125.02,125.10,125.30,125.35,126.10,126.36,126.53,126.67,126.73,127.96,128.10,129.89,129.92,131.15,131.27,131.44,131.58,131.64,131.72,135.84, 136.28,139.73,140.04,140.12,140.19,140.23,140.33,140.37,140.40,140....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com