Synthesis method of 4-allyl-3,5-disubstituted isooxazole
A synthetic method and a two-substitution technology, which is applied in the field of isoxazole preparation, can solve the problems of harsh reaction conditions, low atom utilization rate, and low reaction yield, and achieve the effects of short reaction time, high yield, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
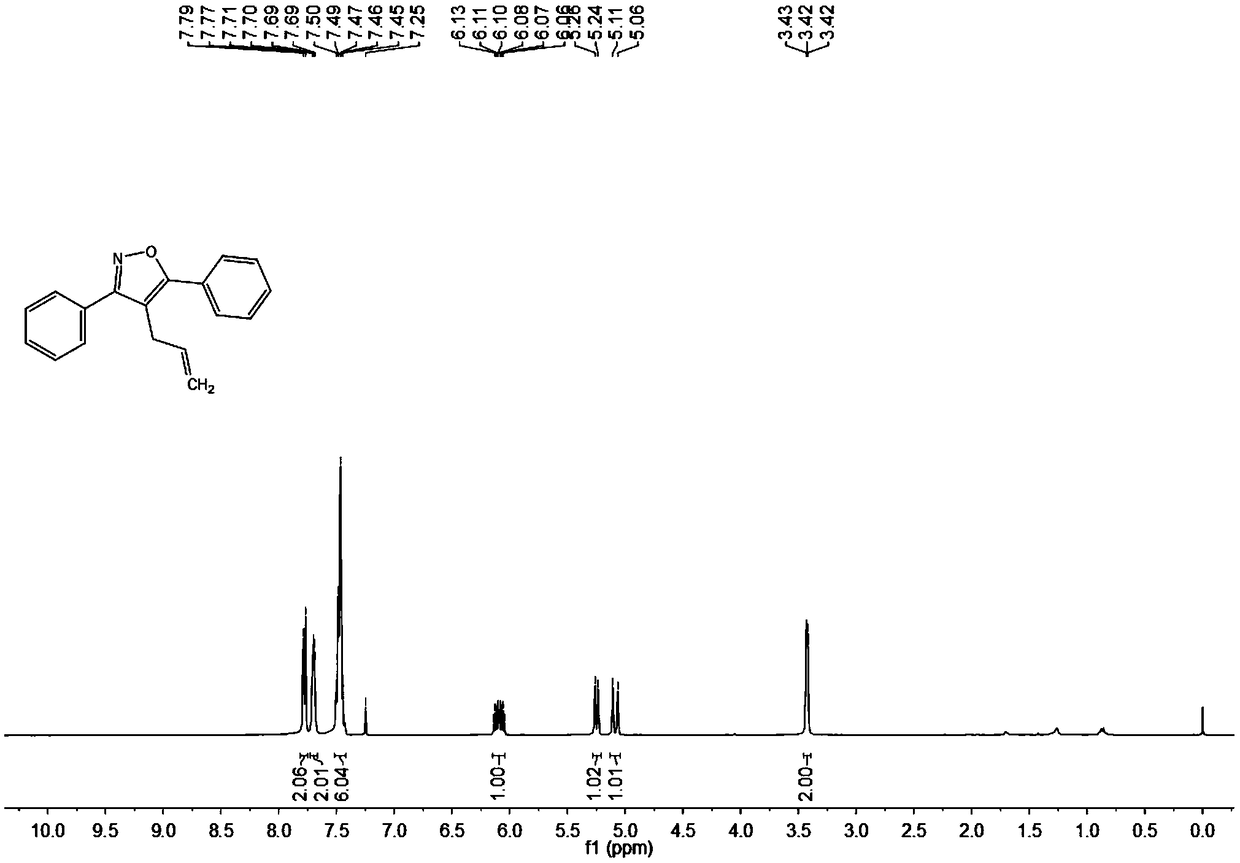
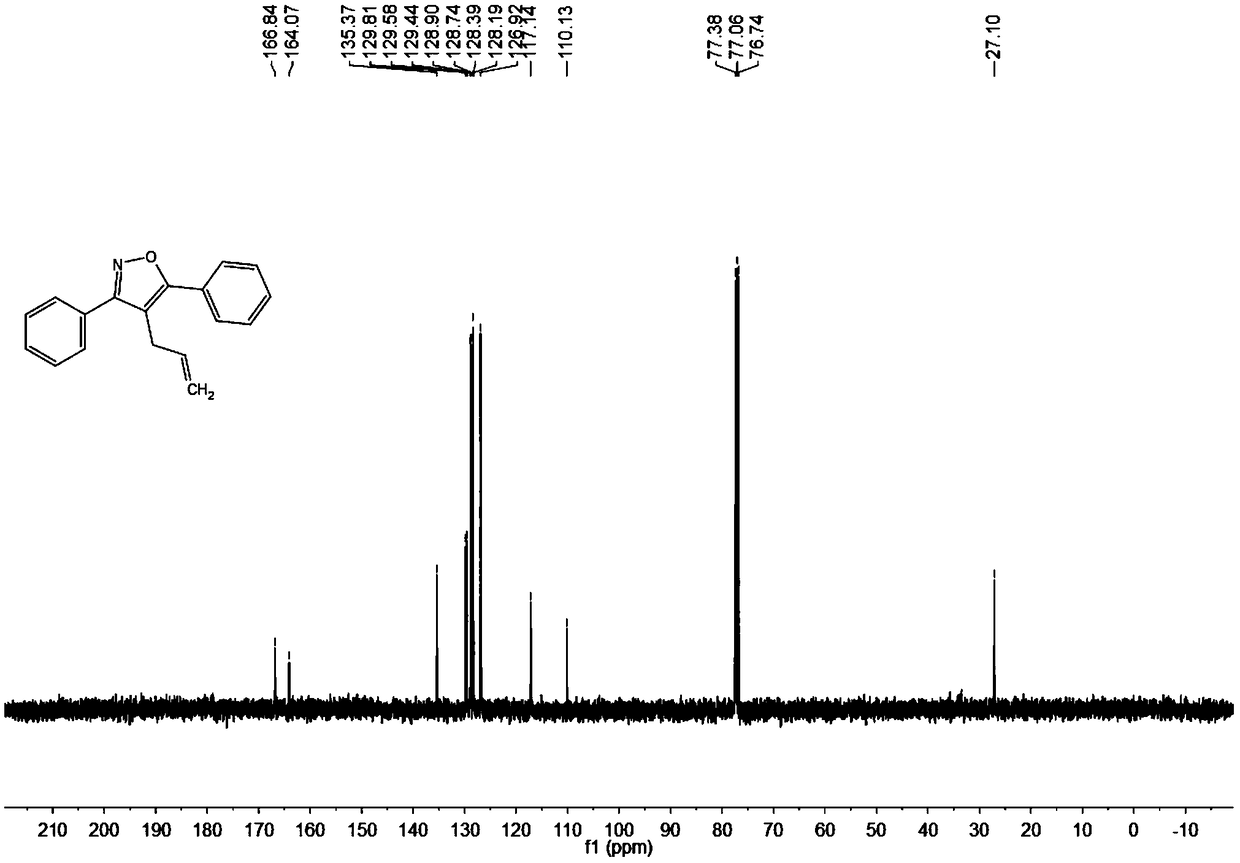
[0053] Add 0.025 mmol Pd(OAc) to the test tube 2 , 0.5 mmol n-butyl ammonium bromide, 0.5 mmol acetylene ketoxime ether (R 1 = R 2 =Ph) and 0.75 mmoles of 3-bromopropene, finally add 1.0 milliliter of N,N-dimethylformamide (DMF) at 600 rpm, stir the reaction at 80°C, detect the reaction by TLC, stop stirring after the reaction is complete, and cool to Add 3 mL of saturated ammonium chloride solution prepared in advance at room temperature, and then add ethyl acetate to extract 3 times to combine the organic phase, and use anhydrous MgSO 4 Drying, filtration, and rotary evaporation under reduced pressure to remove the solvent, using petroleum ether (PE) and ethyl acetate (EA) as developing solvents (PE:EA=200:1), separated by thin-layer plates to obtain the final target product, and the separation yield was 99%. The hydrogen spectrogram and the carbon spectrogram of the product obtained in this embodiment are respectively as follows figure 1 with figure 2shown; the struct...
Embodiment 2
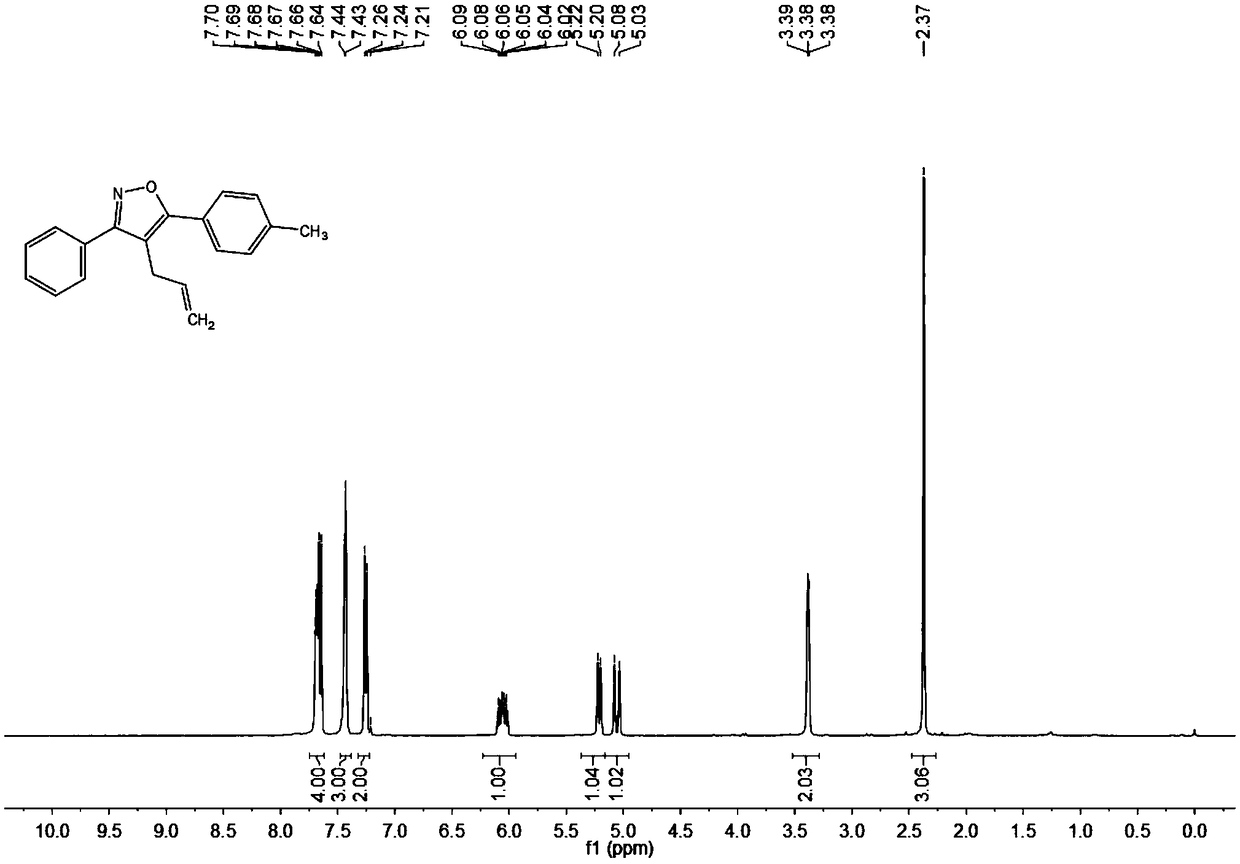
[0061] Add 0.025 mmol Pd(OAc) to the test tube 2 , 0.5 mmol n-butyl ammonium bromide, 0.5 mmol acetylene ketoxime ether (R 1 =Ph,R 2 =4-MePh) and 0.75 mmoles of 3-bromopropene, finally add 1.0 milliliter of N,N-dimethylformamide (DMF) at 600 rpm, stir the reaction at 80°C, detect the reaction by TLC, stop stirring after the reaction is complete, Cool to room temperature and add 3 mL of saturated ammonium chloride solution prepared in advance, then add ethyl acetate to extract 3 times to combine the organic phase, and use anhydrous MgSO 4 Drying, filtration, and rotary evaporation under reduced pressure to remove the solvent, using petroleum ether (PE) and ethyl acetate (EA) as developing solvents (PE:EA=200:1), separated by thin-layer plates to obtain the final target product, and the separation yield was 99%. The hydrogen spectrogram and the carbon spectrogram of the product obtained in this embodiment are respectively as follows image 3 with Figure 4 shown; the struct...
Embodiment 3
[0068] Add 0.025 mmol Pd(OAc) to the test tube 2 , 0.5 mmol n-butyl ammonium bromide, 0.5 mmol acetylene ketoxime ether (R 1 =3,4-diMeOPh,R 2 =Ph) and 0.75 mmoles of 3-bromopropene, finally add 1.0 milliliter of N,N-dimethylformamide (DMF) at 600 rpm, stir the reaction at 80°C, detect the reaction by TLC, stop stirring after the reaction is complete, and cool to Add 3 mL of saturated ammonium chloride solution prepared in advance at room temperature, and then add ethyl acetate to extract 3 times to combine the organic phases, and use anhydrous MgSO 4 Drying, filtration, and rotary evaporation under reduced pressure to remove the solvent, using petroleum ether (PE) and ethyl acetate (EA) as developing solvents (PE:EA=200:1), separated by thin-layer plates to obtain the final target product, and the separation yield was 98%. The hydrogen spectrogram and the carbon spectrogram of the product obtained in this embodiment are respectively as follows Figure 4 with Image 6 show...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com