Carbazole-based carborane derivative material, and preparation method and application thereof
A technology of carborane and its derivatives, which is applied in the field of photoelectric materials, can solve the problems of complex preparation and achieve the effects of simple preparation method, high purity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
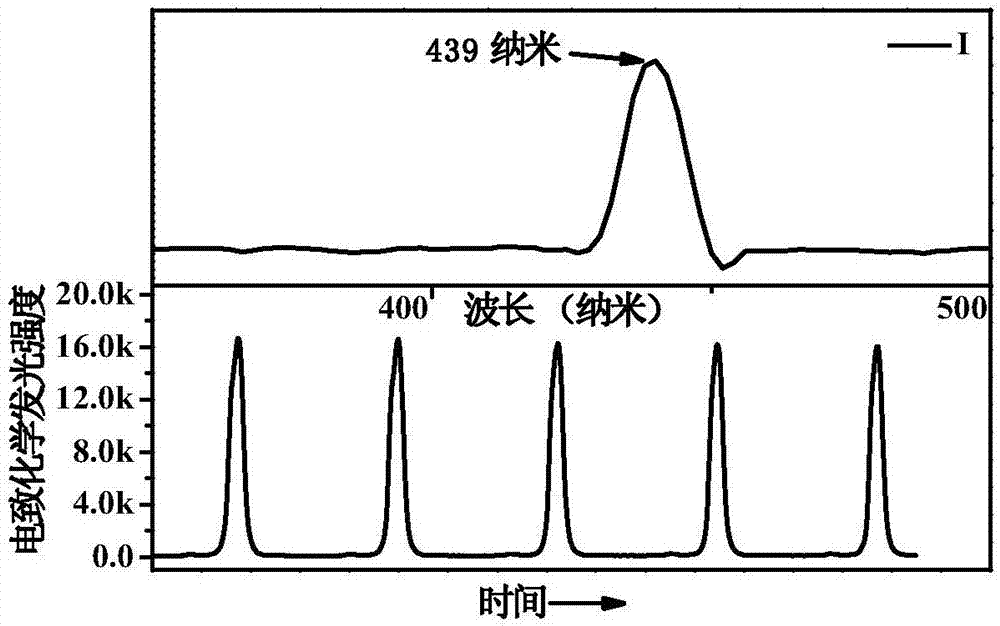
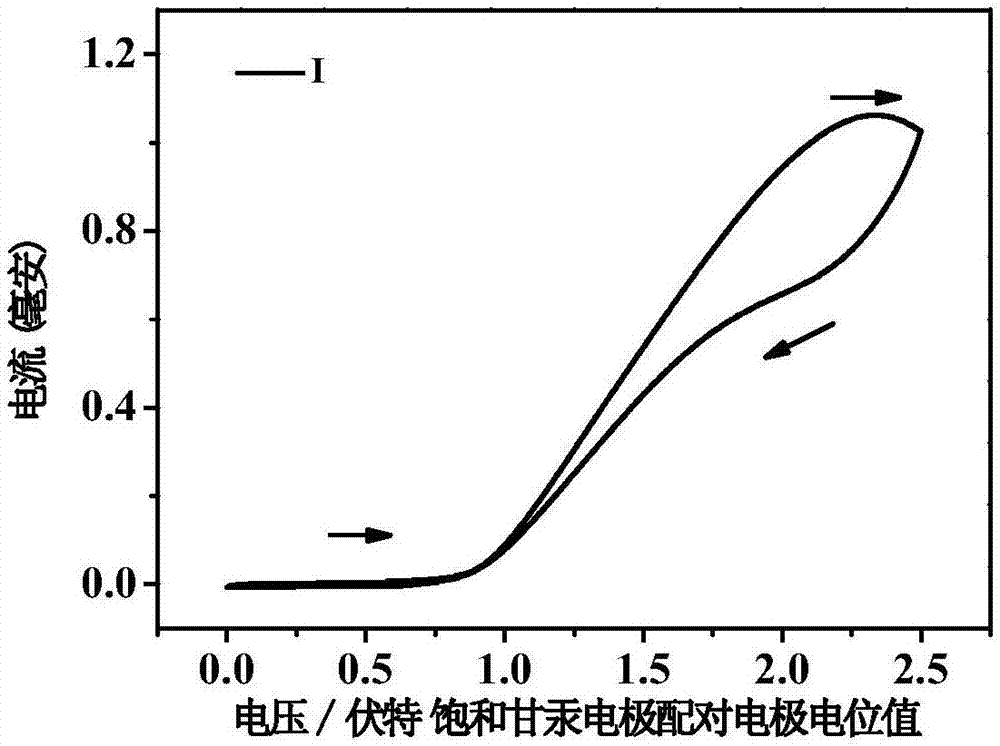
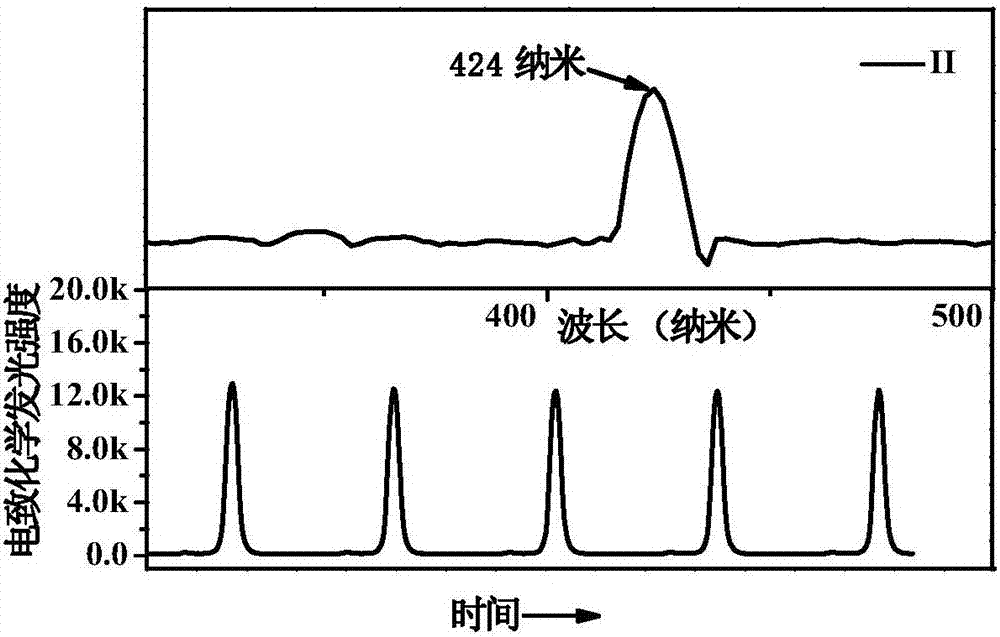
[0031] The preparation method includes: 9-(4-bromophenyl)-9-carbazole, 9-(3-bromophenyl)-9-carbazole obtains 9-(4-ethynylbenzene) through alkynylation of the head coupling reaction -9-carbazole, 9-(3-ethynylbenzene)-9-carbazole, and then react with carborane to synthesize compounds I and II. The carborane derivatives have good applications in electrochemiluminescence, and can be used as small molecule bioluminescent probes. The carborane derivatives have high electrochemiluminescence intensity and good stability.
[0032]
[0033] The preparation method of the carborane derivative material of this type of carbazole comprises the following steps:
[0034] Step 1: Preparation of 9-(4-ethynylbenzene)-9-carbazole and 9-(3-ethynylbenzene)-9-carbazole, with 9-(4-ethynylbenzene)-9-carbazole The preparation of the method is an example: under nitrogen atmosphere and light-shielding conditions, the catalyst of 1 mmol of 9-(4-bromobenzene)-9-carbazole, 0.05-0.1 mmol of tetrakis (trit...
Embodiment 1
[0039]
[0040] Reaction condition 1: under nitrogen and protection from light, 9-(4-bromobenzene)-9-carbazole (322.6 mg, 1.0 mmol), tetrakis(trityl)phosphine palladium (57 mg, 0.05 mmol), cuprous iodide (19 mg, 0.1 mmol) and ethynylbenzene (112 mg, 1.0 mmol) were dissolved in 10 mL of triethylamine (Et 3 N), reflux at 110° C. for 12 hours. After terminating the reaction and cooling to room temperature, the reaction mixture was added to water and extracted with dichloromethane. The remaining organic layer was dried over anhydrous magnesium sulfate and filtered. The solvent was dried by rotary evaporation, and the solid was purified by silica gel column chromatography. Finally, 9-(4-ethynylbenzene)-9-carbazole was obtained as slightly yellow powder (265 mg), with a yield of 61%
[0041] Reaction condition 2: under nitrogen and protection from light, 9-(4-bromobenzene)-9-carbazole (322.6 mg, 1.0 mmol), tetrakis(trityl)phosphine palladium (104 mg, 0.01 mmol), cuprous iodid...
Embodiment 2
[0045]
[0046] Reaction condition one: under the condition of nitrogen protection, decaborane (121.3 mg, 1.0 mmol) and nitrogen, nitrogen-dimethylaniline (181.4 mg, 1.5 mmol) were dissolved in 5 mL of dry distilled toluene solvent, at 35 °C and stirred for 30 minutes. Then 10 mL of the compound 9-(4-ethynylbenzene)-9-carbazole (345 mg, 1.0 mmol) dissolved in distilled toluene was injected, and the reaction mixture was refluxed at 100° C. for 10 hours. After terminating the reaction, the mixture was cooled to room temperature and quenched with methanol. The solvent was removed using rotary evaporation, the residue was added to water, the organic layer was extracted with dichloromethane, dried over anhydrous magnesium sulfate and filtered. The solvent was dried by rotary evaporation and the solid was purified by silica gel column chromatography. Compound I was obtained as a yellow powder, which was recrystallized from methanol to obtain 107 mg of purer Compound I with a yi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com