Symmetric discotic pyrene compounds and preparation method thereof
A compound and symmetrical technology, applied in the preparation of organic compounds, chemical instruments and methods, and the preparation of carboxylic acid nitriles, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
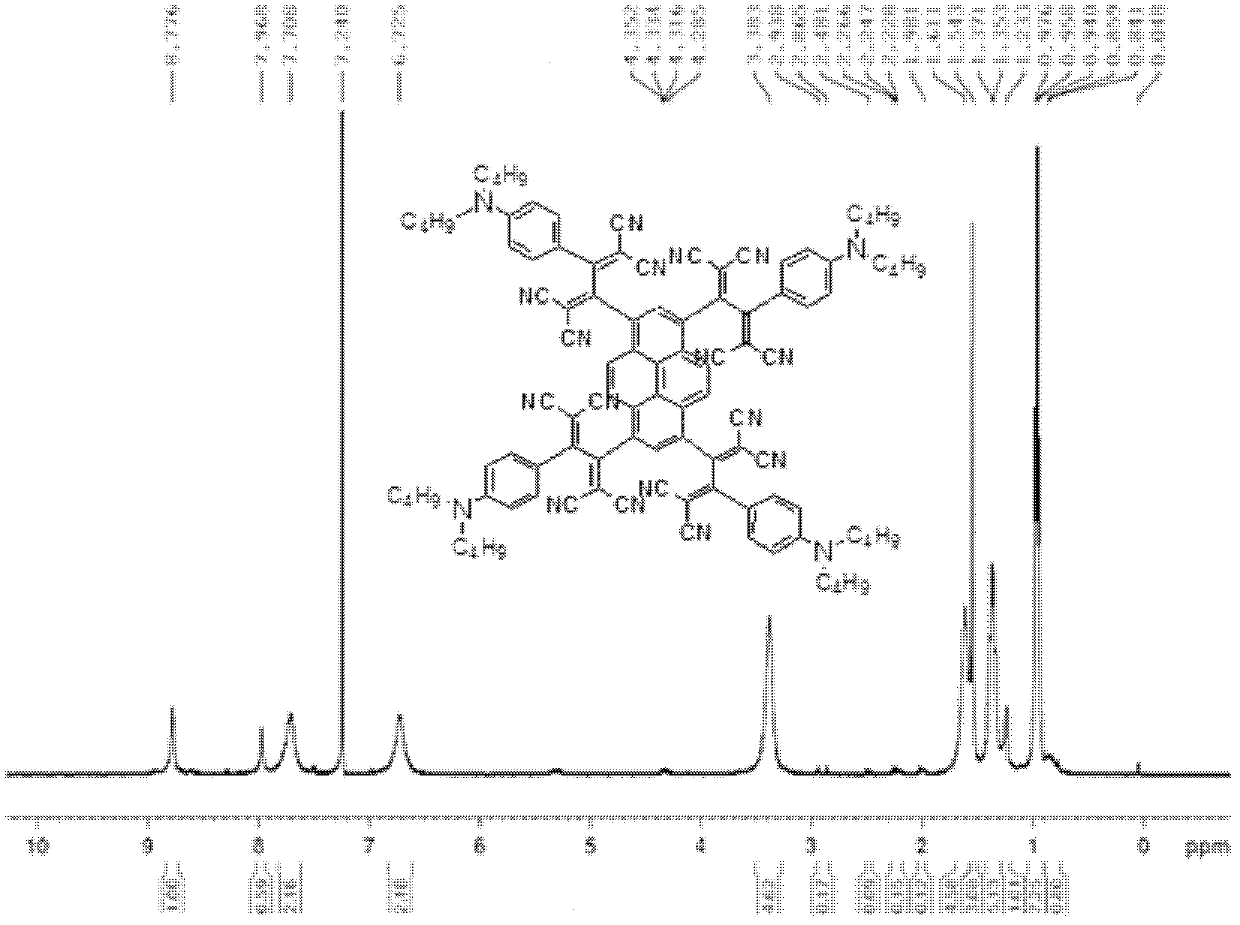
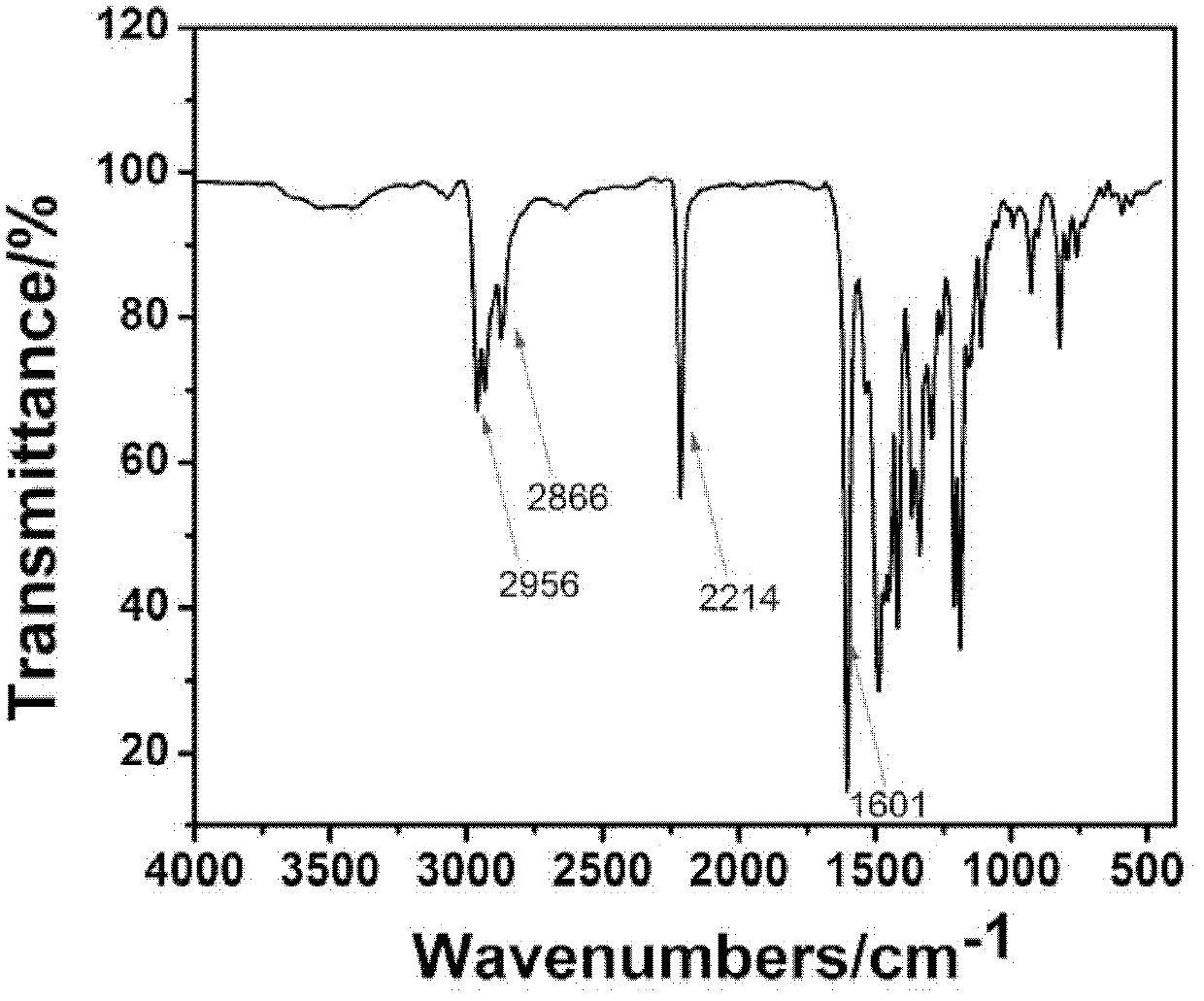
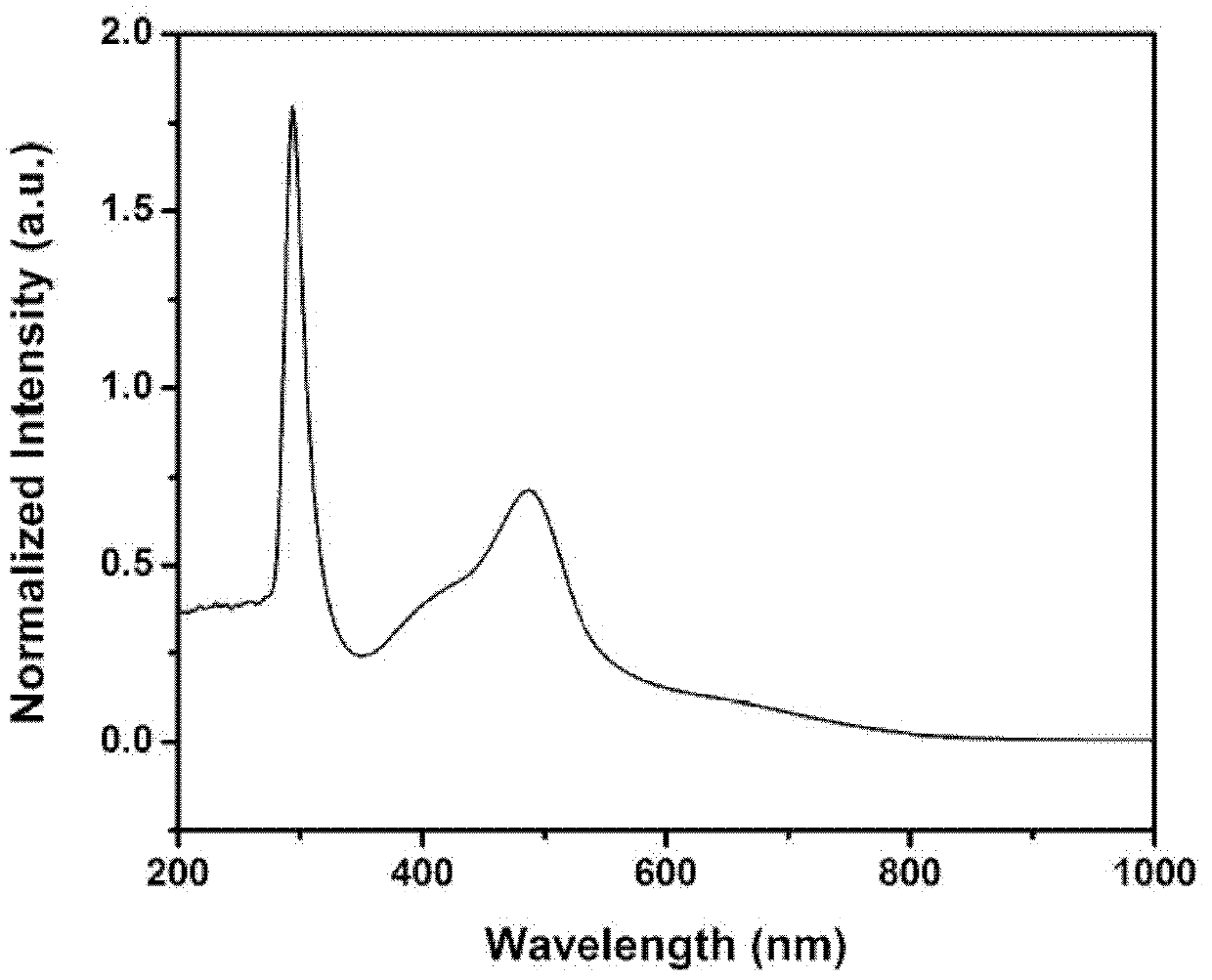
[0031] Embodiment 1: synthetic compound:
[0032]
[0033] with R 1 structured as , R 2 structured as
[0034] The synthetic method of this compound is introduced as an example
[0035] 1: Bromination reaction of pyrene: Dissolve 24.72 mmol pyrene (5.00g) in 250 mL nitrobenzene solution, put it in a 500 mL single-necked flask (with a magnetic stirrer), add excess bromine dropwise to it , the silicone oil bath was raised to 140 degrees Celsius, and the reflux reaction was carried out for 10 hours. After the product is cooled, pour it into dilute sodium bisulfite solution and stir to remove bromine, and change the water every ten minutes until the solution is colorless. The water layer was removed by liquid separation, ethanol was added to the organic layer to continue stirring, suction filtered three times, and then filtered three times with dichloromethane. The filtered product was placed in a vacuum drying oven, and dried to obtain 11.60 g of a light green solid ...
Embodiment 2
[0043] Synthetic compound:
[0044] with R 1 structured as , R 2 structured as The synthetic method of this compound is introduced as an example
[0045] 1: Bromination reaction of pyrene: Dissolve 24.72 mmol pyrene (5.00g) in 250 mL nitrobenzene solution, put it in a 500 mL single-necked flask (with a magnetic stirrer), add excess bromine dropwise to it , the silicone oil bath was raised to 140 degrees Celsius, and the reflux reaction was carried out for 10 hours. After the product is cooled, pour it into dilute sodium bisulfite solution and stir to remove bromine, and change the water every ten minutes until the solution is colorless. The water layer was removed by liquid separation, ethanol was added to the organic layer to continue stirring, suction filtered three times, and then filtered three times with dichloromethane. The filtered product was placed in a vacuum drying oven, and dried to obtain 11.60 g of a light green solid with a yield of 91%.
[0046] 2: ...
Embodiment 3 and 4
[0053]
[0054]
[0055] raw material product Example 3 Example 4 Tetrabromopyrene 0.64g (1.24mM) 0.64g (1.24mM) Trimethylsilylacetylene (TMSA) 0.95g (9.67mM) 0.95g (9.67mM) Dibutyl-p-iodoaniline 2.46g (7.44mM) 2.46g (7.44mM) F4-TCNQ 0.96 g (3.47 mM) —— TTF —— 0.71 g (3.47 mM)
[0056] The final obtained Example 3 was 1.29 g, and Example 4 was 1.07 g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com