Pyrene asymmetrical double-shaft discotic liquid crystal compound and preparation method thereof
A liquid crystal compound, asymmetric technology, applied in the field of discotic liquid crystal compound and its preparation, can solve the problem of few reports, etc., and achieve the effect of wide application prospect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
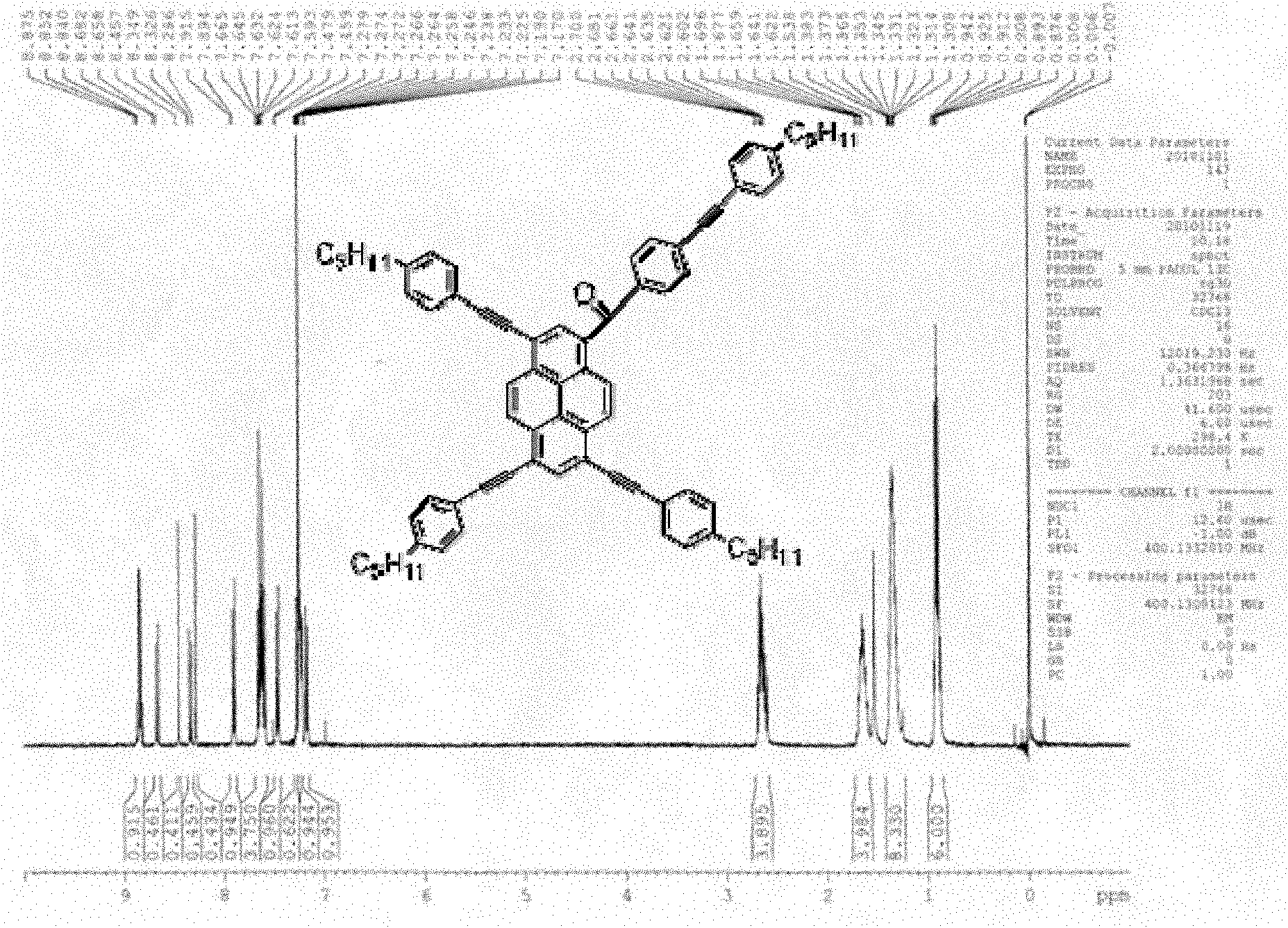
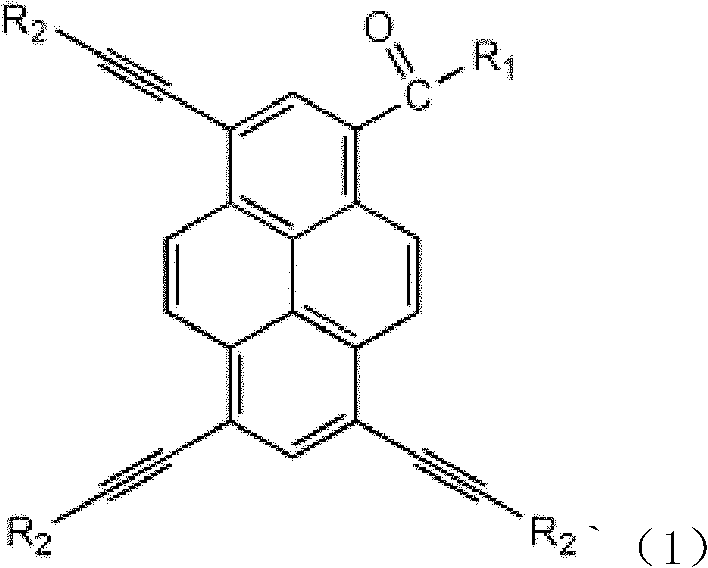
[0020] with R 1 The structure is: R 2 The structure is:
[0021] The synthetic method of this compound is introduced as an example
[0022] Step 1: Friedel-Crafts acylation reaction of pyrene: dissolve 10mmol pyrene (2g) in 30mL carbon disulfide, and place in a 50mL single-necked bottle, add 1.5mmol p-bromobenzoyl chloride (3.26g), and cool in an ice bath to 0 degrees, gradually add 0.02mol aluminum oxide (2.64g); heat and reflux for 12 hours; pour the product into 100mL ice water and stir for 2 hours after cooling, separate the liquid with dichloromethane, and dry and filter the organic phase through anhydrous magnesium sulfate After recrystallization, 2.76 g of p-bromobenzoyl chloride-substituted pyrene was obtained as yellow needle-like crystals, with a yield of 72%.
[0023] Step 2: Bromination reaction of intermediate product: Dissolve 5mmol of p-bromobenzoyl chloride monosubstituted pyrene (1.92g) obtained in step 1 in 50mL of nitrobenzene, place it in a 100mL sin...
Embodiment 2
[0029] with R 1 structured as R 2 The structure is - C 5 h 11
[0030] The synthetic method of this compound is introduced as an example:
[0031] 1: Friedel-Crafts acylation reaction of pyrene: Dissolve 10mmol pyrene (2g) in 30mL carbon disulfide and put it in a 50mL single-necked bottle, add 1.5mmol p-bromobenzoyl chloride (3.26g), and cool to 2.5mL in an ice bath degree, gradually add 0.02mol aluminum oxide (2.64g); heat and reflux for 14 hours; pour the product into 100mL ice water and stir for 3 hours after cooling, separate the liquid with dichloromethane, dry and filter the organic phase through anhydrous magnesium sulfate, and reconstitute Crystallization to obtain 2.76 g of p-bromobenzoyl chloride-substituted pyrene, yellow needle-like crystals, yield 72%;
[0032] 2: Intermediate product bromination reaction: 5mmol p-bromobenzoyl chloride monosubstituted pyrene (1.92g) obtained in step 1 was dissolved in 50mL nitrobenzene, placed in a 100mL single-necked bottl...
Embodiment 3
[0037] with R 1 The structure is:
[0038] R 2 The structure is:
[0039] The synthetic method of this compound is introduced as an example
[0040] 1: Friedel-Crafts acylation reaction of pyrene: Dissolve 10mmol pyrene (2g) in 30mL carbon disulfide and place it in a 50mL single-necked bottle, add 1.5mmol p-bromobenzoyl chloride (3.26g), and cool to 5mL in an ice bath degree, gradually added 0.02mol aluminum oxide (2.64g); heated and refluxed for 16 hours; cooled the product and poured it into 100mL ice water and stirred for 4 hours, separated with dichloromethane, and the organic phase was dried and filtered through anhydrous magnesium sulfate and reconstituted. Crystallization gave 2.76 g of p-bromobenzoyl chloride monosubstituted pyrene, yellow needle-like crystals, and the yield was 72%.
[0041] 2: Intermediate product bromination reaction: 5mmol p-bromobenzoyl chloride monosubstituted pyrene (1.92g) obtained in step 1 was dissolved in 50mL nitrobenzene, placed in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com