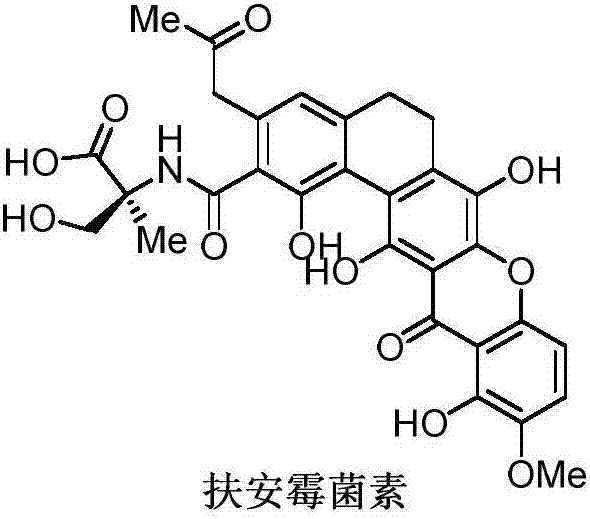
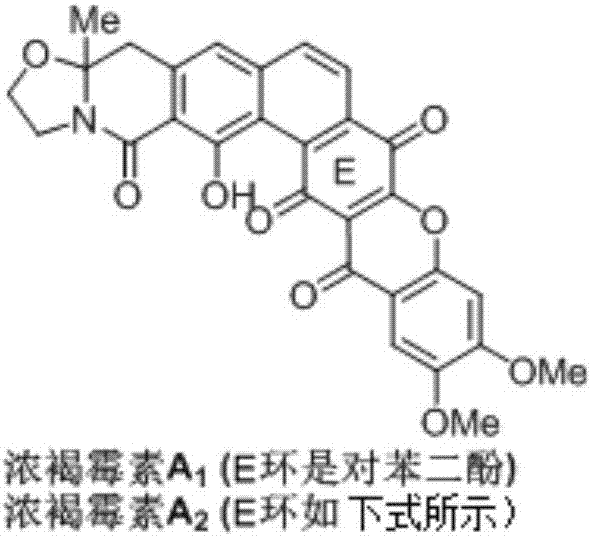
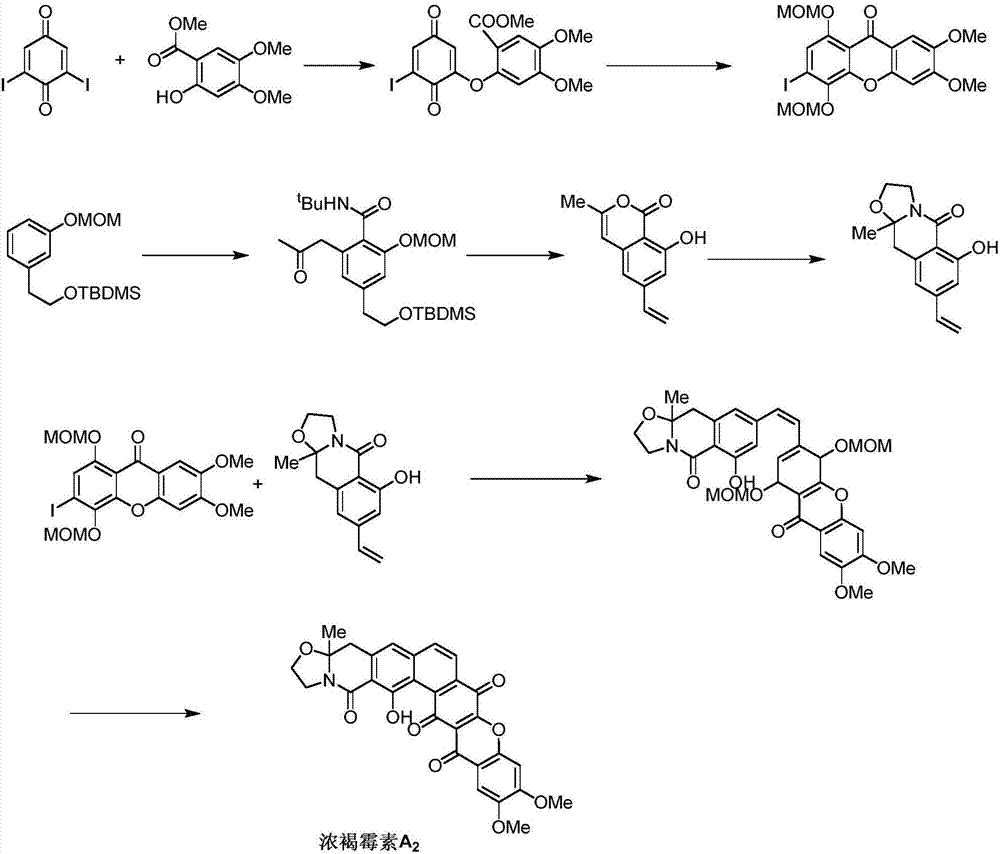
Synthetic method of Fuan mycin skeleton
A technology of Fuanmycin and a synthesis method, which is applied in the synthesis field of synthesizing Fuanmycin skeleton, can solve the problems of low reaction yield, unfavorable mass preparation, low synthesis efficiency and the like, and achieves the effect of simple operation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0044] The present invention is described in further detail and completely below. The presentation includes specific operating and reaction conditions and products. The purity of the product was identified by NMR.
[0045] The synthesis of formula 3 compound:
[0046] Add the compound of formula 2 (16.10g, 48.14mmol) and tetrahydrofuran (160ml) into a 250mL round-bottom bottle, slowly add tetrabutylammonium fluoride (1.0M tetrahydrofuran, 67.4ml, 67,4mmol) at 0°C, add After completion, slowly rise to room temperature and react for 7 hours, and TLC monitors that the reaction of the system is complete. Add 1N hydrochloric acid (100ml) at 0°C, stir for 30 minutes, concentrate and spin off THF, extract 3 times with ethyl acetate, combine the organic phases, wash once with distilled water and once with saturated saline, and wash the organic phase with anhydrous sodium sulfate It was dried, filtered, concentrated, and separated by column chromatography (10% ethyl acetate / petroleu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com