Preparation method of methyl (R)-o-chloromandelate utilizing biocatalytic asymmetric reduction
A technology of methyl mandelate and biocatalysis, which is applied in the field of bioengineering, can solve the problems of increasing energy consumption, increasing costs, and failing to solve the problems of coenzyme regeneration, etc., and achieves the effects of simple operation, high catalytic efficiency, and good industrial application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 Cloning of aldehyde and ketone reductase gene
[0040] According to the gene sequence (Gene ID 937984) of the reductase ytbE of Bacillus subtilis (Bacillus subtilis 168) recorded in Genbank, the PCR primers were designed as follows:
[0041] Upstream primer: CGCGGATCCATGACAACACATTTACAAGCAAAAG;
[0042] Downstream primer: CCGGTCGAGTTAAAAAATCAAAGTTGTCCGGATC.
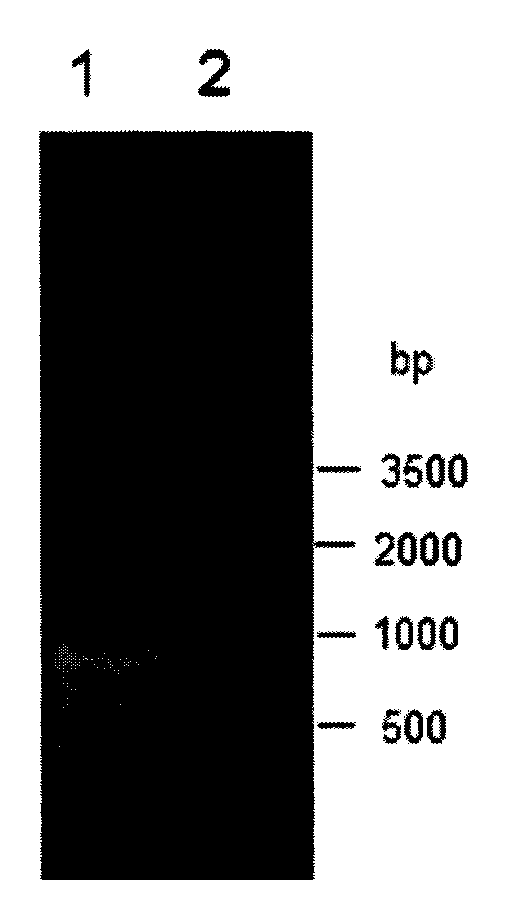
[0043] Wherein, the underlined part of the upstream primer is the BamHI restriction site, and the underlined part of the downstream primer is the XhoI restriction site. PCR amplification was performed using the genomic DNA of Bacillus subtilis 168 (purchased from the Bacillus Genetic Stock Center, Ohio State University, USA, BGSC) as a template. The PCR system is: 15 μl of 2×Taq PCR MasterMix, 1 μl (0.3 μmol / L) of each upstream primer and downstream primer, 1 μl (0.1 μg) of DNA template and ddH 2 O 12 μl. PCR amplification steps are: (1) 95°C, pre-denaturation for 5min; (2) 94°C, denaturation for 45s; ...
Embodiment 2
[0044] Example 2 Preparation of recombinant plasmid pET28a-AKR
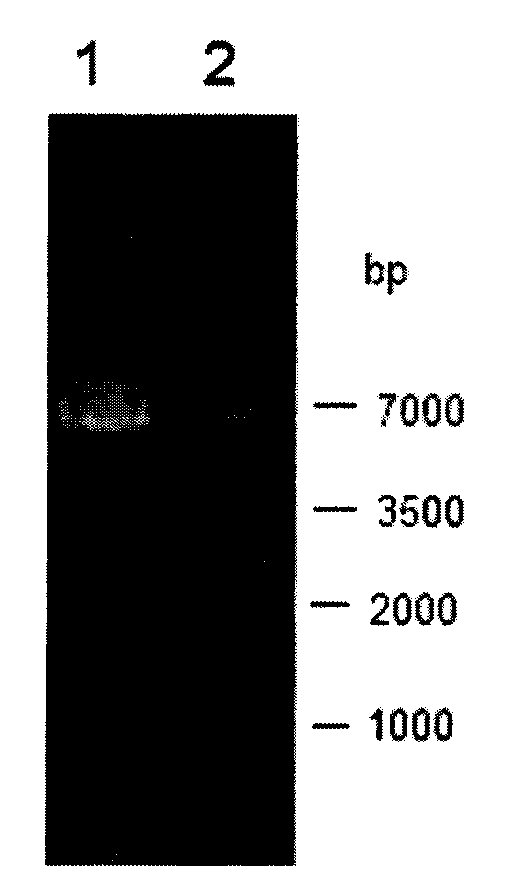
[0045] The aldehyde and ketone reductase gene target band recovered in Example 1 was double-digested with restriction endonucleases BamHI and XhoI at 37°C for 12 hours, purified by agarose gel electrophoresis, and recovered using an agarose gel DNA recovery kit target fragment. Under the action of T4 DNA ligase, the target fragment was ligated with the plasmid pET28a digested with BamHI and XhoI at 4°C overnight to obtain the recombinant plasmid pET28a-AKR.
Embodiment 3
[0046] Example 3 Cloning of Glucose Dehydrogenase Gene
[0047] According to the glucose dehydrogenase gene sequence (Gene ID 938261) of Bacillus subtilis (Bacillus subtilis) 168 included in Genbank, design specific primers:
[0048] Upstream primer: TGGTGGTGGTGGTGCTTAACCGCGGCCTGCCTGGAA;
[0049] Downstream primer: ACTTTGATTTTTAACAAGGAGATATACATATGTATCC.
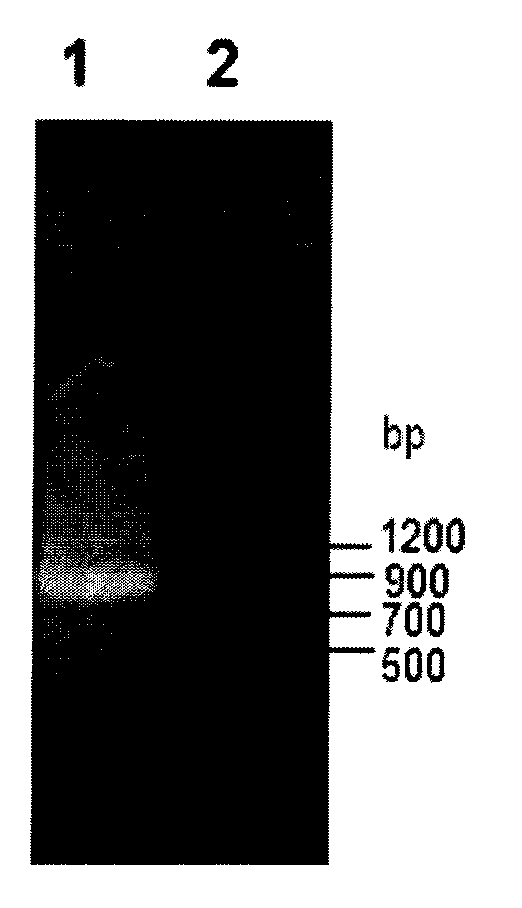
[0050] Using the genomic DNA of Bacillus subtilis 168 as a template, PCR amplification was performed. The PCR system is: 15 μl of 2×Taq PCR MasterMix, 1 μl (0.3 μmol / L) of each upstream primer and downstream primer, 1 μl (0.1 μg) of DNA template and ddH 2 O 12 μl. PCR amplification steps are: (1) 95°C, pre-denaturation for 5min; (2) 94°C, denaturation for 45s; (3) 57°C annealing for 1min; (4) 72°C extension for 1min; steps (2) to (4) repeated 35 times; (5) Continue to extend at 72°C for 10 minutes, then cool to 4°C. The PCR product was purified by agarose gel electrophoresis, and the target band in the interval of 700-900 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| enantiomeric excess | aaaaa | aaaaa |
| enantiomeric excess | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com