A preparation method of clopidogrel hydrogen sulfate type II
A technology of clopidogrel bisulfate and clopidogrel free base, applied in the direction of organic chemistry, can solve the problems of large solvent usage, high cost, cumbersome operation, etc., save time for solvent concentration, improve recycling, The effect of reducing the generation of waste liquid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
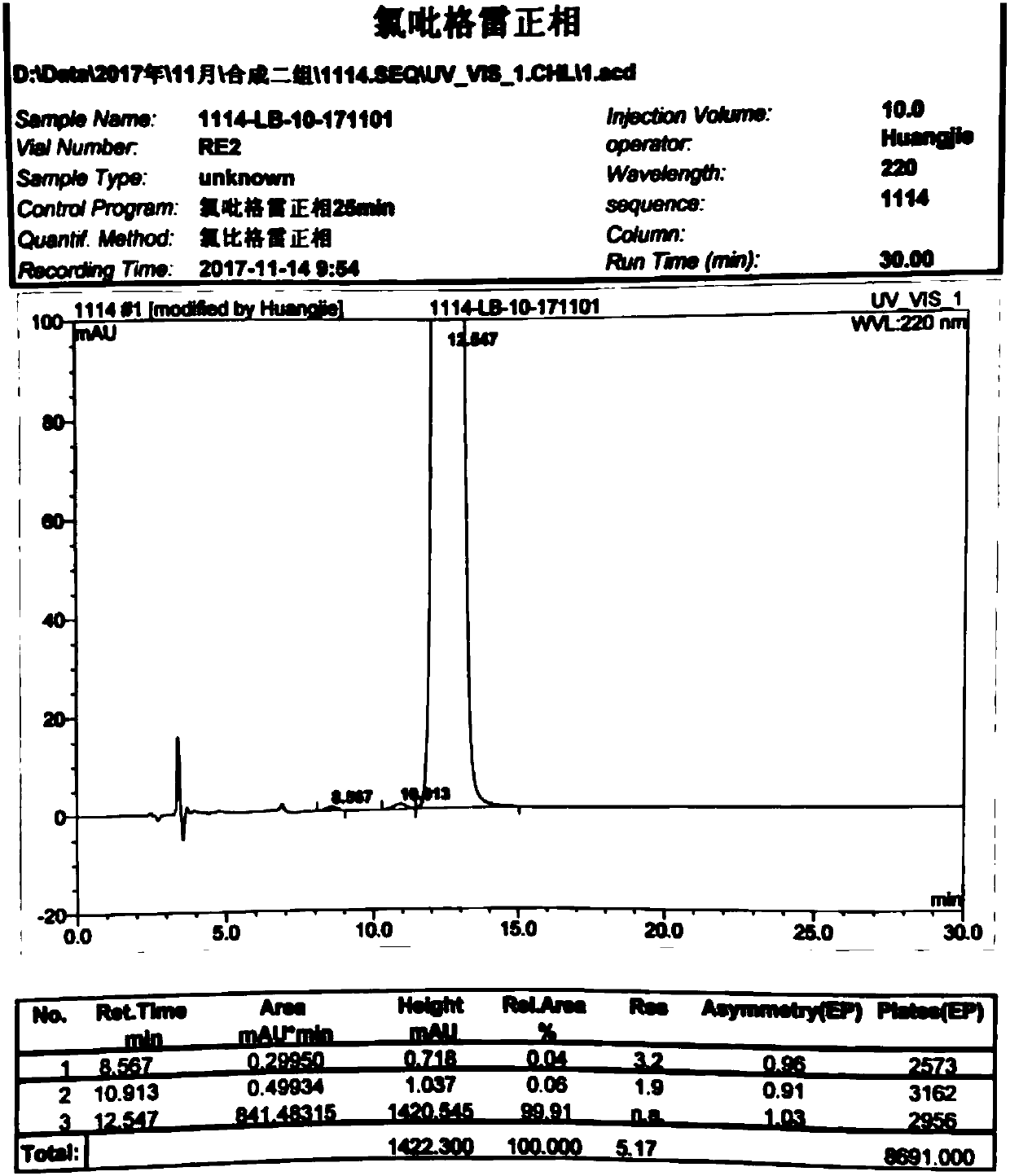
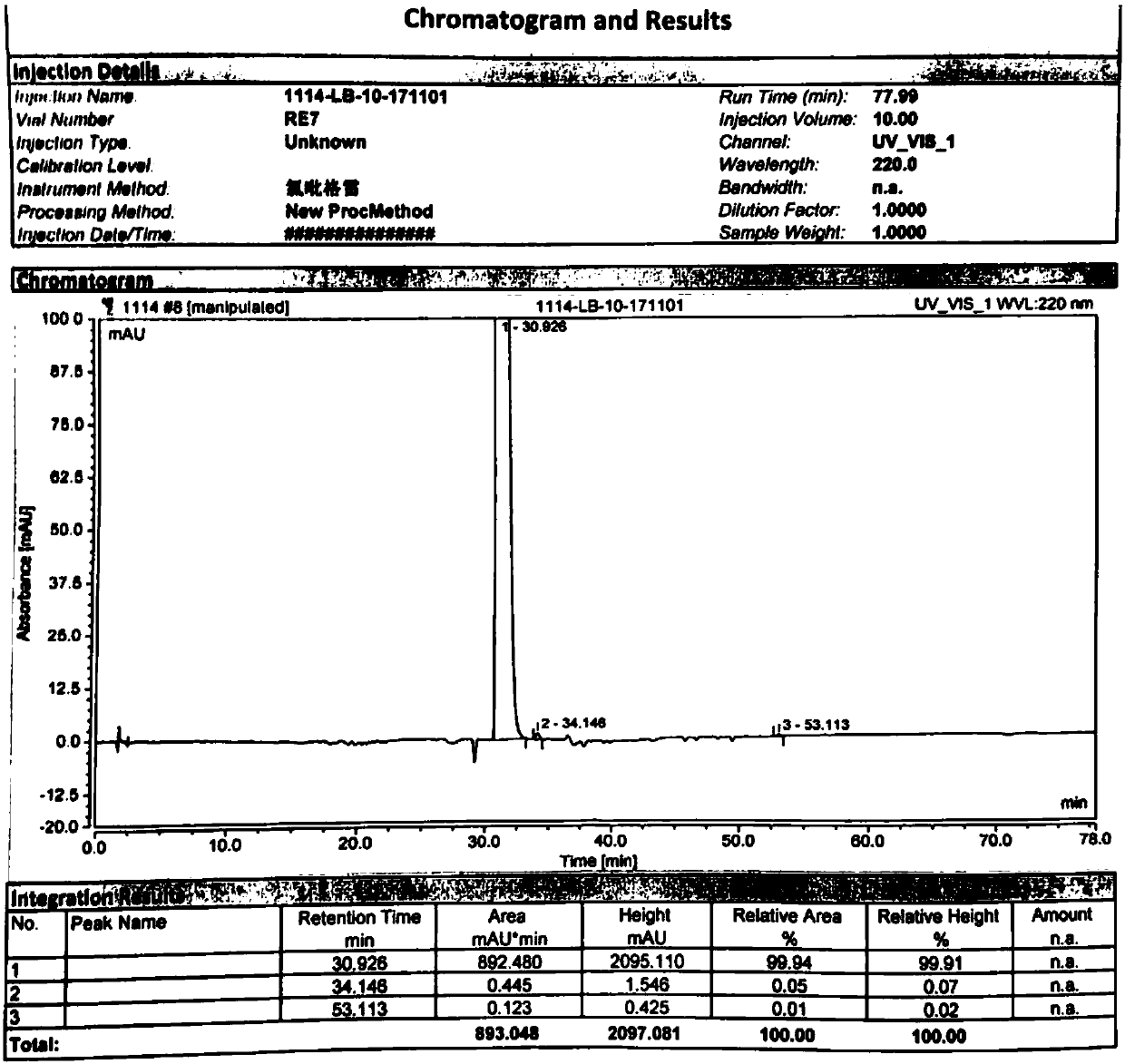
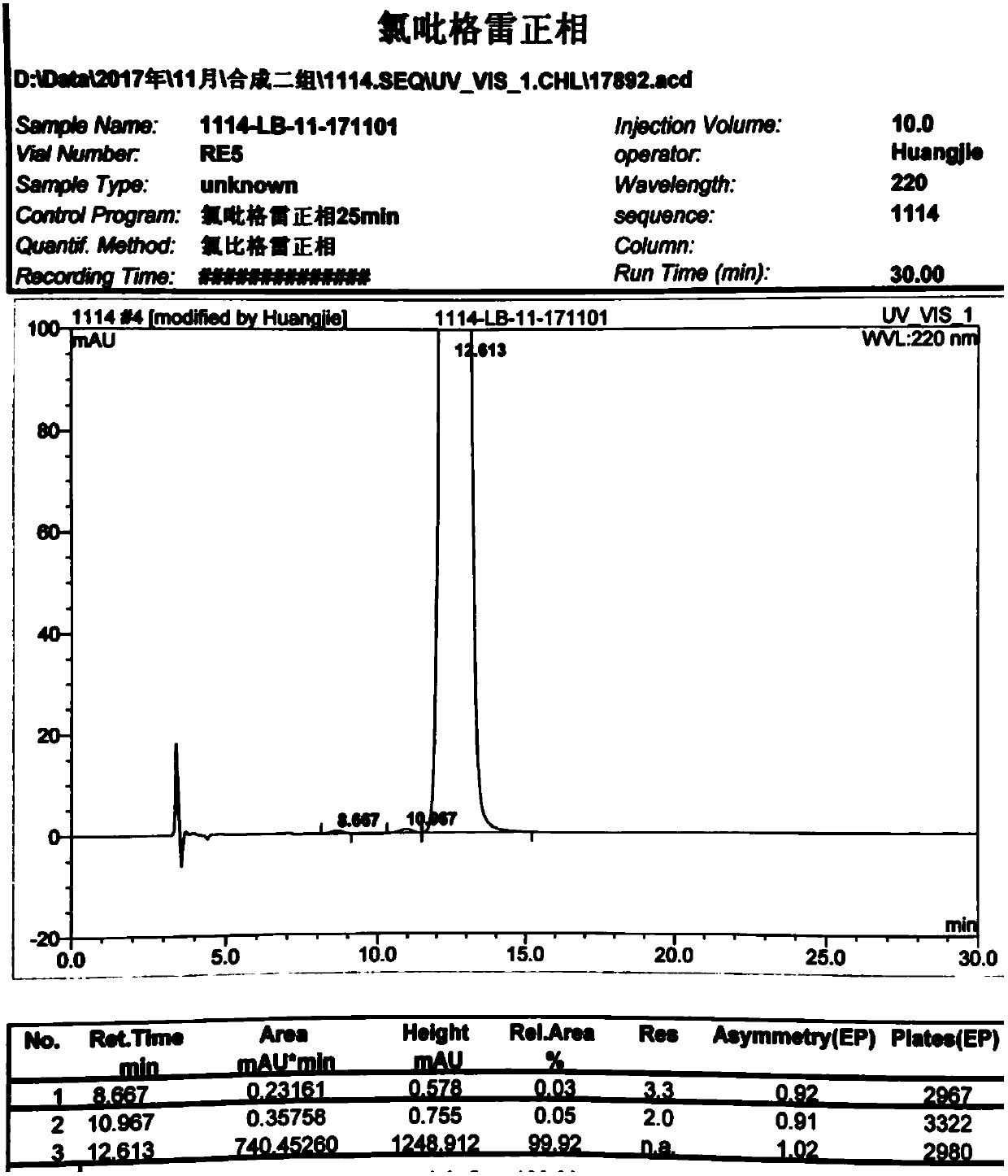
Image
Examples
Embodiment 1
[0115] From o-chloromandelic acid to type II clopidogrel bisulfate, the specific preparation method is as follows:
[0116] The first step: the preparation of R-o-chloromandelic acid methyl ester
[0117] Add 200.0g of o-chloromandelic acid, 397.5g of dichloromethane, and 51.6g of methanol into a 1L three-necked flask, stir and dissolve at 15-25°C, slowly pour 20.0g of concentrated sulfuric acid, heat up to 42°C, keep warm in a water bath for 3 hours, and cool to room temperature, washed with purified water, separated the organic phase, and washed with 5% NaHCO 3 Adjust the organic layer to neutral, wash the organic phase with water, and dry over anhydrous sodium sulfate. Suction filtration to obtain R-o-chloromandelic acid methyl ester solution, the yield was determined by external standard method: 96.3%, and the purity: 99.57%. 1 H-NMR (400MHz, CD 3 OD), δ: 3.314 (3H, d), 5.605 (1H, s), 7.274-7.340 (2H, dd), 7.370-7.422 (1H, m), 7.480-7.523 (1H, m).
[0118] The second s...
Embodiment 2
[0132] Investigate the impact of solvent feed ratio and reaction temperature on product purity and yield in the preparation process of R-o-chloromandelic acid methyl ester, see Table 1, Table 2. Others are the same as embodiment 1.
[0133] The influence of table 1 solvent charging ratio on product yield and purity
[0134]
[0135] As seen from Table 1, in above-mentioned six groups of experiments, under the situation that temperature of reaction, reaction time, concentration temperature, concentration time are constant, the mol ratio of R-o-chloromandelic acid, methyl alcohol, organic solvent, the vitriol oil is 1: When 1.5:4.3:0.2, the product yield purity that obtains is the highest.
[0136] The impact of table 2 reaction temperature on product yield and purity
[0137]
[0138] It can be seen from Table 2 that in the above six groups of experiments, when the feed ratio, reaction time, concentration temperature and concentration time are constant, the product yiel...
Embodiment 3
[0140] Investigate the impact of catalyst consumption, temperature of reaction and reaction time in the process of 2-benzenesulfonic acid group-2 (2-chlorophenyl) methyl acetate preparation, product purity and yield, see table 3, table 4, table 5
[0141] The influence of table 3 catalyst (DMAP) charging ratio on product yield and purity
[0142]
[0143]
[0144] As seen from Table 3, in above-mentioned seven groups of experiments, under the situation that temperature of reaction, dropwise addition time, reaction time, concentration time are constant, methyl mandelate: organic base: catalyst: the mol ratio of benzenesulfonyl chloride is 1: When 1:0.075:1.0, the product yield and purity obtained are the highest.
[0145] The impact of table 4 benzenesulfonyl chloride charging ratio on product yield and purity
[0146]
[0147] As can be seen from Table 4, in the above-mentioned 4 groups of experiments, under the situation that temperature of reaction, dripping time, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com