Separation extraction method for (R)-2-chloromandelic acid
A technology of o-chloromandelic acid and extraction method, applied in the separation/purification of carboxylic acid compounds, organic chemistry, etc., can solve the problems of high market price and unsatisfied market demand, and achieve simple operation, simple equipment and long service life long lasting effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
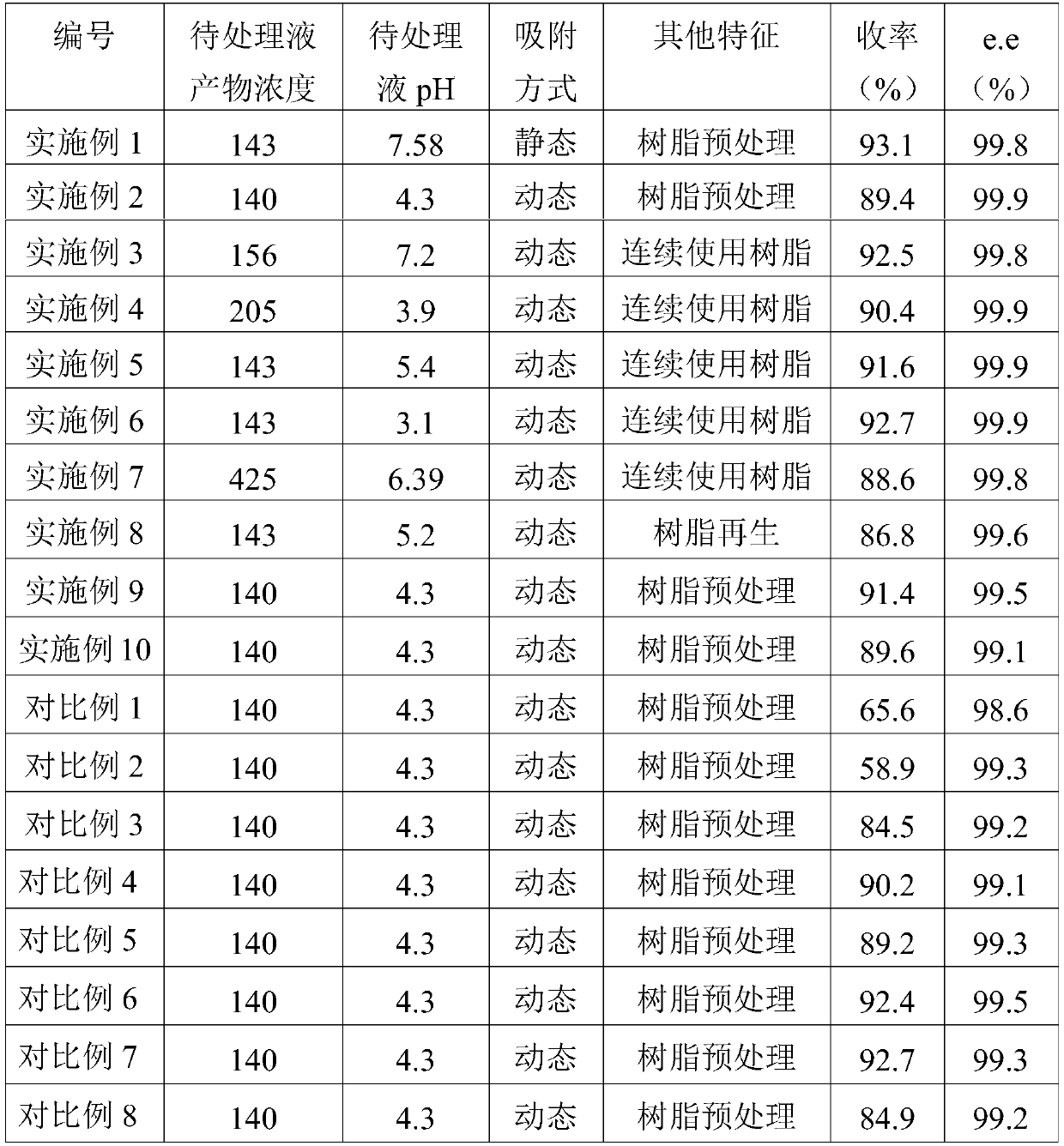
[0088] The pH=7.58, (R)-o-chloromandelic acid content of 143mmol / L water-phase immobilized bacteria catalytic reaction liquid is the liquid to be treated, using HZ-202 type resin.
[0089] 1. Pretreatment under the conditions of 25°C and 220rpm shaker: first soak and wash with 1M NaOH solution, the ratio of the volume of NaOH solution (in ml) to the mass of strongly basic anion resin (in g) is 3: 1. Then soak and wash with 1M dilute hydrochloric acid. The ratio of the volume of dilute hydrochloric acid (unit: ml) to the mass (unit: g) of strong basic anion resin is 3:1. washing;
[0090] 2. Soak the treated resin with a buffer solution of pH=8, extract the product o-chloromandelic acid from the reaction solution by static adsorption, hang the column for a certain period of time (2-6 hours), and separate the liquid from the resin;
[0091] 3. Elute the adsorbed resin with 1M dilute hydrochloric acid 5 times the mass volume of the resin at a flow rate of 2.5ml / min;
[0092] 4....
Embodiment 2
[0094] pH = 4.3, (R)-o-chloromandelic acid content of 140mmol / L water-phase immobilized bacteria catalytic reaction liquid is the liquid to be treated, using HZ-202 resin.
[0095] 1. Pretreatment at room temperature: first soak and wash with 1M NaOH solution, the ratio of the volume of NaOH solution (in ml) to the mass of strongly basic anion resin (in g) is 3:1, and then use 1M dilute hydrochloric acid For soaking and washing, the ratio of the volume of dilute hydrochloric acid (in ml) to the mass of strongly basic anion resin (in g) is 3:1, soak for 15 minutes, and wash at a speed of 2 ml / min;
[0096] 2. Soak the treated resin with a buffer solution of pH=8, extract the product o-chloromandelic acid from the reaction solution by means of dynamic adsorption, and after hanging on the column for 15 minutes, separate the liquid from the resin at a rate of 2.5ml / min;
[0097] 3. Elute the adsorbed resin with 1M dilute hydrochloric acid 5 times the mass volume of the resin at a ...
Embodiment 3
[0100] The water-phase free bacteria catalyzed reaction liquid with pH=7.2 and (R)-o-chloromandelic acid content of 156mmol / L is the liquid to be treated, and HZ 202 resin is used.
[0101] 1. In the exchange column at room temperature, the resin that has been recycled once is used for adsorption, and the product o-chloromandelic acid is extracted by dynamic adsorption. After hanging on the column for 15 minutes, the liquid is separated from the resin at a speed of 2.5ml / min;
[0102] 2. Elute the adsorbed resin with 1M dilute hydrochloric acid 5 times the mass volume of the resin at a flow rate of 2.5ml / min;
[0103] 3. The collected desorption solution is extracted with ethyl acetate of 0.3 times the volume of the eluent, and the extracted organic phase is distilled under reduced pressure (50°C-60°C, 0.09MPa), and the obtained solid is vacuum-dried to obtain (R )-o-chloromandelic acid crude product. The experimental results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com