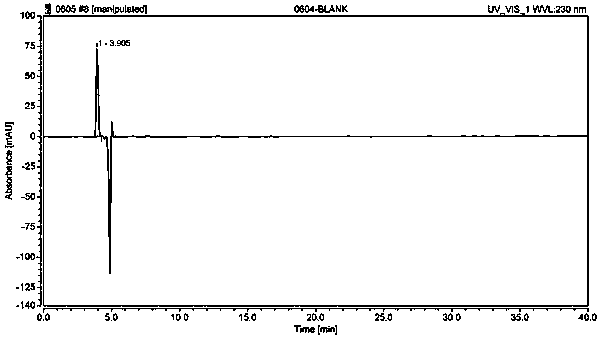
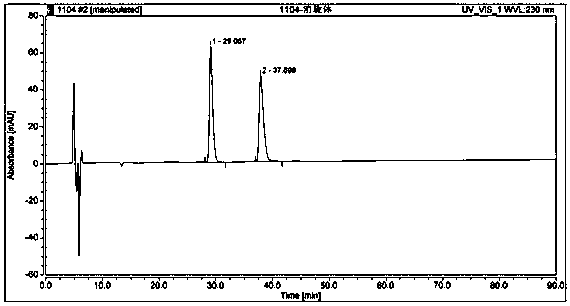
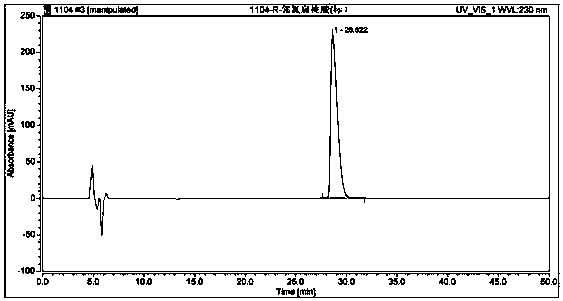
Method used for separating and measuring 2-chloromandelic acid enantiomers via HPLC
A kind of technology of o-chloromandelic acid and enantiomer, applied in the field of medicine and chemical industry, and can solve the problems such as the inability to achieve drug effect and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] A method for separating and determining o-chloromandelic acid enantiomers by HPLC, comprising the steps of:
[0037] (1) For the preparation of the detection solution:
[0038] Preparation of system suitability solution: Take about 10 mg of o-chloromandelic acid racemate reference substance, accurately weigh it into a 25mL volumetric flask, dissolve it with a diluent and dilute to the mark, shake well, and use it as a system suitability solution.
[0039] Preparation of standard solution: Take about 10 mg of R-o-chloromandelic acid standard, accurately weigh it into a 25mL volumetric flask, dissolve it ultrasonically with a diluent and dilute to the mark, shake well, and use it as a standard solution.
[0040] Preparation of the test solution: Take an appropriate amount of self-made R-o-chloromandelic acid (for the specific production method, refer to the patent "A Method and Application of Enzymatic Preparation of R-o-chloromandelic acid", application number: 201510978...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com