Preparation method of lanoconazole
A technology of lanoconazole and suranoconazole, which is applied in the field of preparation of lanoconazole, can solve the problems of unstable reaction and low yield, improve yield and purity quality, avoid excessive impurities, Guarantee the effect of stable reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041]
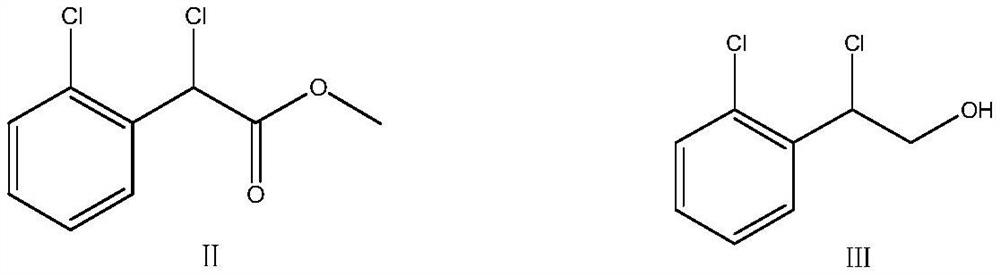
[0042] 150±5kg of anhydrous methanol was pumped into the reactor, stirring was started, 20kg (107.2mol) of 2-chloromandelic acid and 0.5kg of concentrated sulfuric acid were added, the hot water valve was opened, the temperature was raised to 65° C. for reflux reaction for 3h, and the reaction ended. After cooling down to room temperature, add 10% sodium hydroxide aqueous solution dropwise to the reaction solution to adjust the pH value to near neutrality, after stabilization, then heat up to 50 ℃~60 ℃ to carry out vacuum distillation to recover methanol solvent When it is dry, add 20±2kg of dichloromethane to the residue, stir and mix without uniformity, obtain a light yellow liquid viscous substance containing compound VI of dichloromethane, and directly proceed to the next step.
Embodiment 2
[0044]
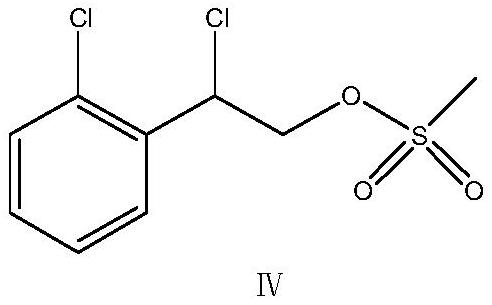
[0045] The dichloromethane solvent of 200 ± 5kg was pumped into the reactor, stirring was started, the compound VI obtained in the above-mentioned embodiment 1 was completely dissolved, the basic catalyst DMF of 0.4kg was added, and the temperature was raised to 40~45 ℃ under stirring. Add the mixed solution of 16.3kg (128.6mol) of oxalyl chloride and 20±2kg of dichloromethane in a clean and dry high-level tank, and the temperature of the dropwise addition process is controlled within this range. After the dropwise addition, the reflux reaction is continued for 1h. , the reaction solution is slowly cooled down to room temperature, and then slowly add cold water 50 ± 5kg to the reaction kettle, and then use a mass percentage of 20% Na 2 CO 3 Aqueous solution 70 ± 5kg, adjust the pH value of the reaction solution to 6 ~ 7, stir for 30 minutes, stand for stratification, collect the organic phase, the upper layer aqueous phase can be extracted once with 50 ± 5kg of dic...
Embodiment 3
[0047]
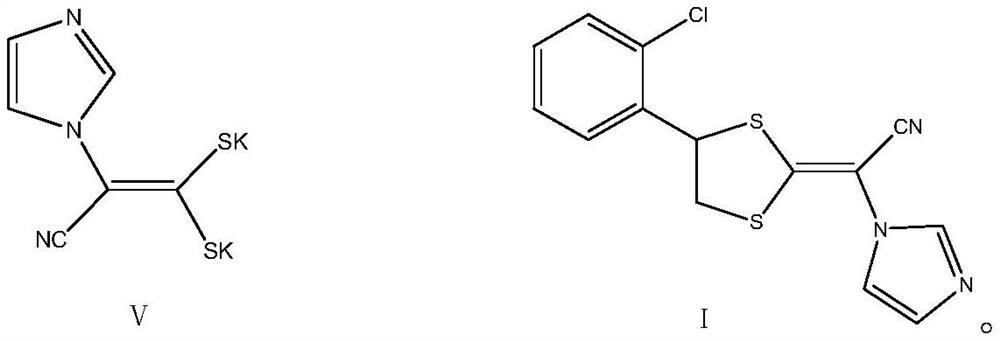
[0048] In the yellow liquid compound II that above-mentioned embodiment 2 obtains, add 200 ± 5kg methanol solvent, carry out cooling and cooling, the reaction liquid system is cooled to 0~5 ℃, add potassium borohydride again in batches and the total amount is 14.4kg ( 268mol), control the feeding in 2~3h, and then control the temperature at 0~5℃ under ice bath cooling to continue the reaction for 2h. After the reaction, let the system temperature reach room temperature and continue to stir the reaction for 2h. After the end, use the mass percentage as 10% dilute hydrochloric acid was used to adjust the pH of the system to neutrality, then the temperature was raised to 50-60°C, and the methanol solvent was evaporated under reduced pressure to recover the methanol solvent, and about 100 ± 5kg was evaporated. Analyze the crystal, centrifuge, remove the white solid salt, wash with 20 ± 2kg methanol, the filtrate continues to concentrate under reduced pressure, steam abo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com