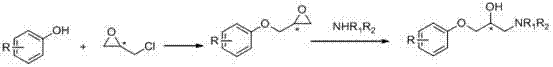
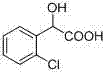
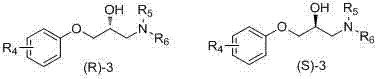Resolution method for 2-chloromandelic acid by crystalizing diastereomeric salts
A technology of o-chloromandelic acid and diastereomers, applied in the field of medicinal chemistry, can solve the problems of small scale, few resolutions, and expensive resolution agents, and achieve the effects of easy separation, low cost, and simple synthesis methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1 R in the general formula II 1 =o-CH 3 , R 2 =-CH 3, R 3 =-CH 3 The (S / R)-2 (S / R)-N,N-dimethyl-1-o-tolyloxy-3-amino-2-propanol is the chiral resolving agent, and R in the general formula III 4 =o-OCH 3 , R 5 =-CH 3, R 6 =-CH 3 Resolution of (S / R)-3 (S / R)-N,N-dimethyl-1-o-methoxyphenoxy-3-amino-2-propanol as a nucleation inhibitor for racemization o-chloromandelic acid 1.
[0049] 1. Step (1)
[0050] 18.6g (0.1mol) racemic o-chloromandelic acid, 18.8g (0.09mol) (S) -2 , 2.25g (0.01mol) (S)-3 in 150ml of ethanol, magnetically stirred at 30°C for 5-6 hours, then left to stand, until solid precipitated, suction filtered after 6 hours to obtain a white solid R -1 -S -2 20.9g (0.053mol, yield 82% (calculated based on the amount of single-configuration o-chloromandelic acid)).
[0051] 2. Step (2)
[0052] The solid R obtained by suction filtration in the first step -1 -S -2 Dissolve 20.9g in 100ml of water, add 26.5ml of dilute sulfuric acid a...
Embodiment 2
[0053] Embodiment 2: R in general formula II 1 =-H, R 2 =-C 2 h 5, R 3 =-C 2 h 5 The (S / R)-2 (S / R)-N,N-diethyl-1-phenoxy-3-amino-2-propanol is the chiral resolving agent, and R in the general formula III 4 =o-NO 2 , R 5 =-CH 3, R 6 =-C 2 h 5 Resolution of (S / R)-3 i.e. (S / R)--N-methyl-N-ethyl-1-o-nitrophenoxy-3-amino-2-propanol as a nucleating inhibitor Racemic o-chloromandelic acid 1.
[0054] 1. Step (1)
[0055] Add 18.6g (0.1mol) of racemic o-chloromandelic acid, 11.2g (0.05mol) of (S)-2, 12.7g (0.05mol) of (S)-3, and 150ml of ethyl acetate into a 250ml round bottom flask , stirred magnetically at 30°C for 5-6 hours, then stood still, and after 6 hours, suction filtered to obtain a white solid R -1 -S -2 15.5g (0.038mol, yield 76% (calculated based on the amount of single-configuration o-chloromandelic acid)).
[0056] 2. Step (2)
[0057] The solid R obtained by suction filtration in the first step -1 -S -2 Dissolve 15.5g in 50ml of water, add 19ml o...
Embodiment 3
[0059] Embodiment 3: R in general formula II 1 = o-Cl, R 2 =-C 2 h 5 , R 3 =-CH 3 The (S / R)-2 (S / R)-N-methyl-N-ethyl-1-o-chlorophenoxy-3-amino-2-propanol is a chiral resolving agent, the general formula R in III 4 =o-NH 2 , R 5 =-C 2 h 5, R 6 =-C 2 h 5 Resolution of (S / R)-3 (S / R)-N,N-diethyl-1-o-aminophenoxy-3-amino-2-propanol as a nucleation inhibitor for racemic o Chloromandelic acid 1.
[0060] 1. step one)
[0061] Add 18.6g (0.1mol) of racemic o-methylmandelic acid, 17.07g (0.07mol) of (S)-2, 7.1g (0.03mol) of (S)-3, and 150ml of isopropanol into a 250ml round bottom flask , stirred magnetically at 90°C for 3-4 hours, then stood still, and after 6 hours, suction filtered to obtain a white solid R-1-S-2 17.2g (0.04mol, yield 80% (in a single configuration Calculation of the amount of o-chloromandelic acid)).
[0062] 2. Step (2)
[0063] The solid R obtained by suction filtration in the first step -1 -S -2 Dissolve 17.2g in 50ml of water, add 38ml o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com