Preparation method of R-o-chloromandelic acid and alcohol ester thereof
A technology of o-chloromandelic acid and amino acid, applied in the field of bioengineering, can solve problems such as high cost and complicated production process, and achieve the effects of low cost, short reaction time and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: The preparation method of R-o-chloromandelic acid comprises the following steps:
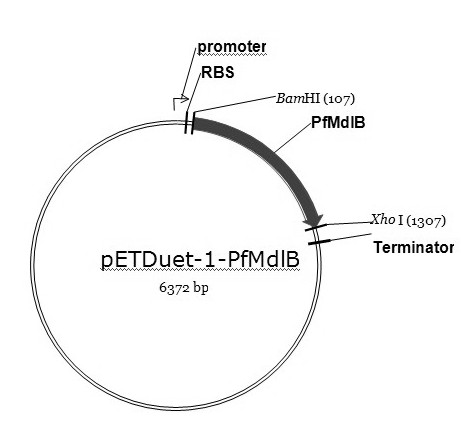
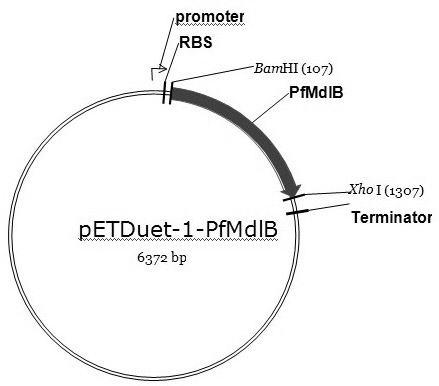
[0037] (1) Recombinant expression of enzymes
[0038] Design primer pair P1, P2:
[0039] P1: 5'-atacgcggatccatgtggaacaatgctccgc3', P2: 5'-gggccgctcgagttaacttctcgcttgttc3',
[0040] Pseudomonas fluorescens ( Pseudomonas fluorescens ) genomic DNA as a PCR template for PCR amplification, the PCR system is: 10×Taq buffer 5μl; 10 mmol / L dNTP 1.5μl; 25 mmol / L MgCl 2 3μl; 1μl each of upstream and downstream primers; 1μl DNA template; 5U / μl Taq DNA polymerase 0.25μl; ddH 2 O 37.25μl; PCR amplification steps are: (1) 95°C, pre-denaturation for 3min; (2) 94°C, denaturation for 30s; (3) 50°C annealing for 30s; (4) 72°C extension for 75s; repeat step (2) ~(4) 30 times; (5) Continue to extend at 72°C for 10min, then cool to 4°C. The PCR product was purified by agarose gel electrophoresis, and the target band in the 1100-1200bp interval was recovered by using the agarose gel DNA rec...
Embodiment 2
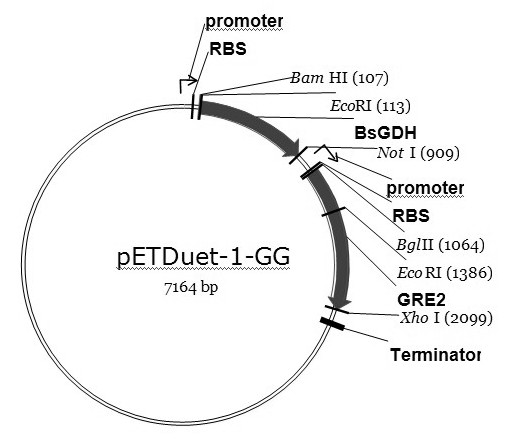
[0052] Example 2: The preparation method of R-methyl o-chloromandelate is substantially the same as Example 1, comprising the following steps:
[0053] Esterify racemic o-chloromandelic acid with methanol under the action of concentrated sulfuric acid to produce racemic o-chloromandelic acid methyl ester. The following steps are the same as steps (1), (2), (3) and (4) in Example 1, and finally R-methyl o-chloromandelate is obtained.
[0054] The preparation of other alcohol esters only needs to change the esterified alcohol into the corresponding alcohol.
[0055]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com