Method for synthesizing novel uric acid lowering compound Arhalofenate intermediate
A synthetic method and technology for reducing uric acid, which is applied in the separation/purification of carboxylic acid compounds, the preparation of organic compounds, organic chemical methods, etc., can solve the problems of long synthesis reaction time, harsh reaction conditions, and high price, and meet the production conditions The effect of low equipment requirements, high split rate, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
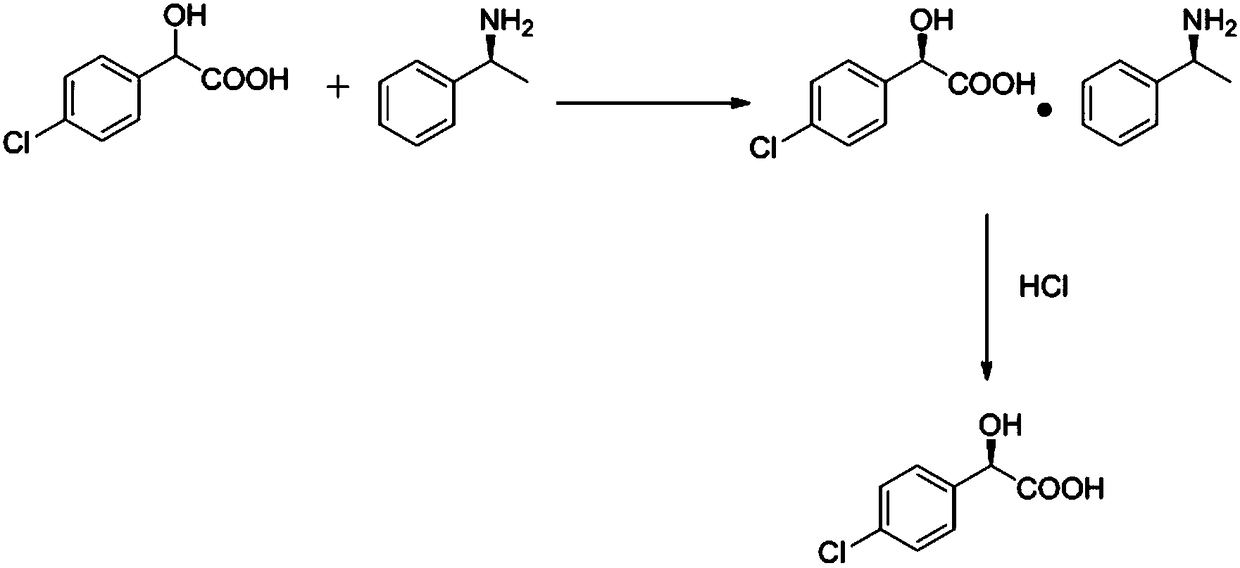
[0021] A method for synthesizing a novel uric acid-reducing compound Arhalofenate intermediate, wherein the novel uric acid-reducing compound Arhalofenate intermediate is (R)-p-chloromandelic acid. The above-mentioned (R)-p-chloromandelic acid is an important chiral intermediate and is used to synthesize drugs. It can be used as an acidic resolving agent to resolve the new uric acid-lowering compound Arhalofenate, improve the yield and resolution of the compound Arhalofenate The compound Arhalofenate has the disadvantages of long reaction time, harsh reaction conditions, low yield, high price and so on, so that the compound Arhalofenate will become an important anti-gout drug.
[0022] The synthetic method of novel uric acid-lowering compound Arhalofenate intermediate, its reaction formula is:
[0023]
[0024] The synthetic method of novel uric acid-lowering compound Arhalofenate intermediate, its synthetic concrete steps are:
[0025] Step 1: Add 95% ethanol to the racem...
Embodiment 2
[0030] The synthetic method of novel uric acid-lowering compound Arhalofenate intermediate, its synthetic concrete steps are:
[0031] Step 1: Add 95% ethanol to the racemic p-chloromandelic acid at a ratio of 1:10 (g / mL) to dissolve it completely. The above-mentioned 95% ethanol solution contains 0.035% N,N- Diisopropylethylamine, the presence of N,N-diisopropylethylamine can increase the number of hydrogen bonds formed between the amino group on the (R)-amine and the carboxyl group on the (R)-p-chloromandelic acid, and then The formation of a hydrogen bond network is conducive to increasing the stability of the crystal structure of the insoluble salt (R)-amine·(R)-p-chloromandelic acid, thereby increasing the stability difference between the two salts, while the insoluble salt (R)- The hydrogen bonds in the crystal structure of amine·(R)-p-chloromandelic acid are connected by the Cl...Cl halogen bonds that appear, reducing the insoluble salt of (R)-amine·(R)-p-chloromandelic...
Embodiment 3
[0036] The synthetic method of novel uric acid-lowering compound Arhalofenate intermediate, its synthetic concrete steps are:
[0037] Step 1: Put 2.8g of p-chloromandelic acid in a 100mL single-necked bottle, add 30mL of 95% ethanol solution, stir until completely dissolved, then add 1.9mL of (R)-phenylethylamine dropwise under stirring , mixed and heated to reflux for 15 minutes to dissolve the solid, then slowly cooled to room temperature, and filtered to obtain 2.413 g of crude product;
[0038] Step 2: The crude product obtained in step 1 was recrystallized twice in 95% ethanol to obtain 1.25 g of (R)-phenethylamine·(R)-p-chloromandelic acid;
[0039] Step 3: Dissolve the (R)-phenethylamine·(R)-p-chloromandelic acid obtained in step 1 in 16 mL of water, add hydrochloric acid to adjust the pH of the solution to 1, then extract three times with ethyl acetate, combine the organic phases, After drying over anhydrous sodium sulfate, the solvent was distilled off under reduced...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com