Process for synthesizing antithrombin inhibitor of non-asymmetric non-peptide kind
An antithrombin and non-peptide technology, which is applied in the field of synthesis of new antithrombin inhibitors, can solve the problems of low total yield, high price, and low yield, and achieve high total yield and short route , the effect of separating simple
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
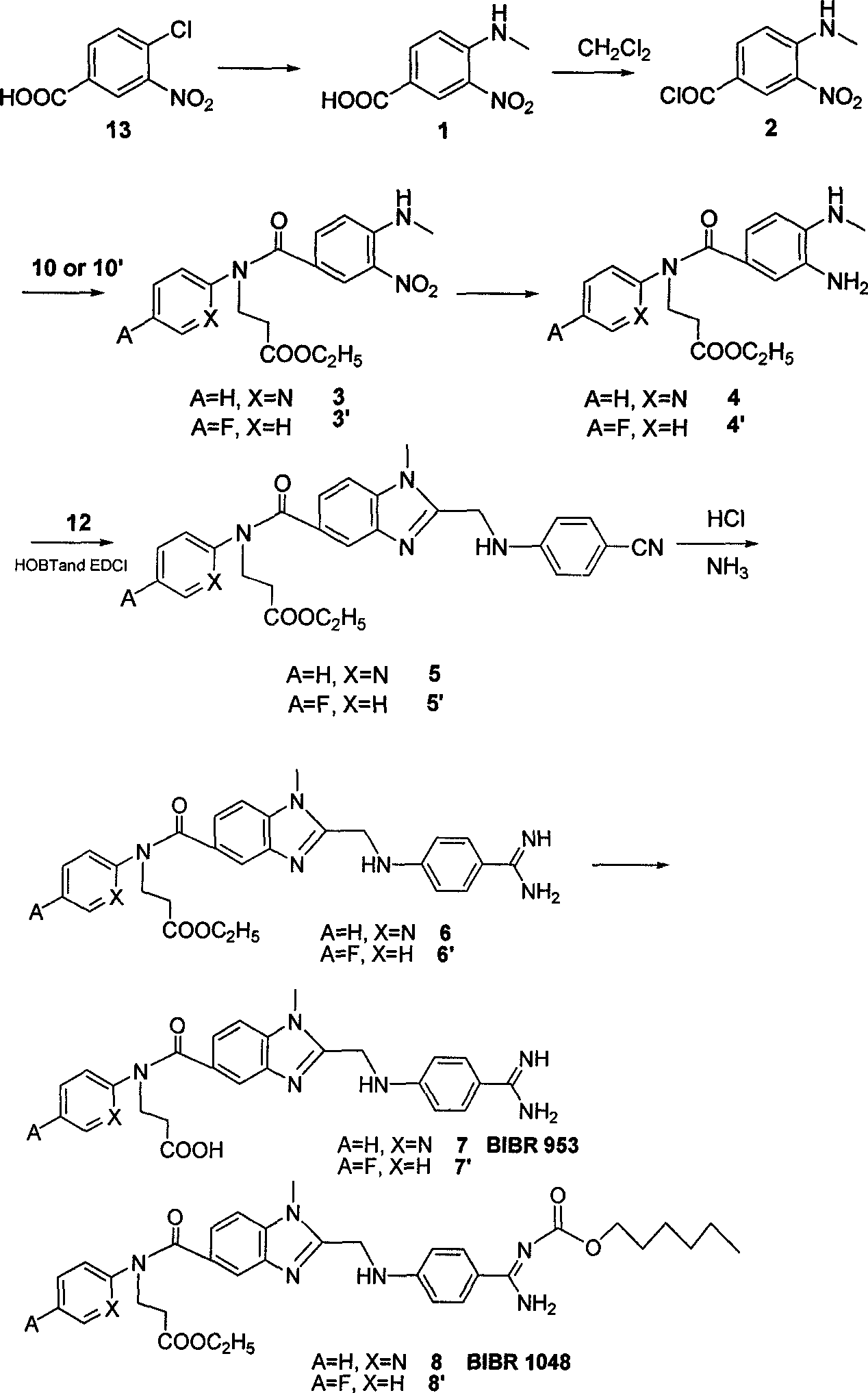
Embodiment 1
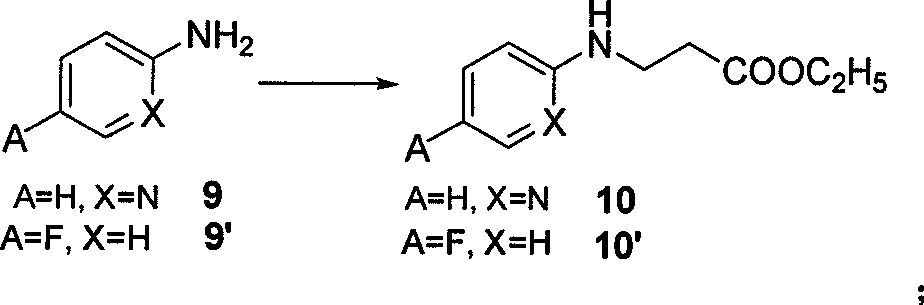
[0039] Synthesis of ethyl 3-(pyridine-2-imine)-propionate (10)
[0040]Under the protection of nitrogen, add ethyl acrylate (27.5 g, 0.275 mol) to compound 9 (A=H, X=N) - 2-aminopyridine (22.5 g, 0.25 mol), stir and reflux at higher than 100 ° C After 24 hours, the precipitate was filtered off, and the residue was concentrated and purified by a silica gel column to obtain a white solid 10 (38 g, 72%).
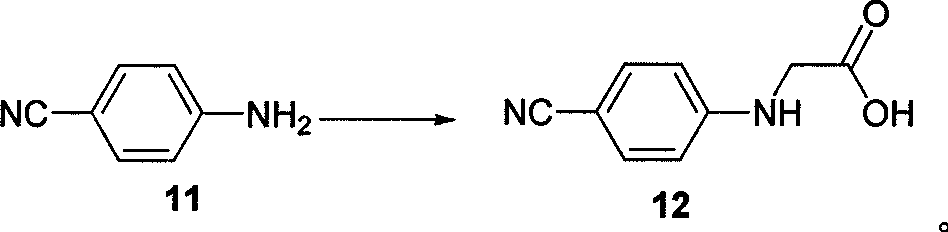
[0041] Synthesis of 1-(4-cyano-phenylimine)-acetic acid (12)
[0042] Add 150ml of water to compound 11 (6.0g, 0.05mol) and 1-chloroacetic acid (10g, 0.11mol) and heat to reflux until a large amount of yellow solid precipitates, filter at room temperature, and rinse with water, absolute ethanol, and anhydrous ether, respectively. After washing, yellow solid 12 (6.4 g, 73%) was obtained.
[0043] Synthesis of 4-aminomethyl-3-nitro-benzoic acid (1)
[0044] 150ml of 25%-30% methylamine aqueous solution was added to compound 13 (25g, 0.124mol), and the system was reacted at a t...
Embodiment 2
[0060] Synthesis of ethyl 3-(4-fluorophenyl-1-imine)-propionate (10')
[0061] Under nitrogen protection, add ethyl acrylate (27.5 g, 0.275 mol) to compound 9' (A=F, X=H) - 4-fluoroaniline (23.5 g, 0.25 mol), add 10 ml of absolute ethanol and 10 ml of Triethylamine was stirred and refluxed at higher than 100°C for 24h, the precipitate was filtered off, and the residue was concentrated and purified by a silica gel column to obtain a light red solid 10' (43g, 77%).
[0062] Synthesis of 3-[(4-aminomethyl-3-nitro-benzoyl)-(4-fluorobenzene)-2-imine]-propionic acid ethyl ester (3')
[0063] Compound 10' (10.5g, 0.05mol) was dissolved in 30ml CH 2 Cl 2 and 30ml triethylamine, slowly add the CH of compound 2 at room temperature 2 Cl 2 solution. The mixed system was reacted at room temperature for 12 h, the precipitate was filtered off, and the residue was concentrated and purified by a silica gel column to obtain a yellow oily liquid 3' (19.0 g, 98%).
[0064] Synthesis of 3-[(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com